Abstract
Background
Despite improving survival rates, children are at risk for long-term cognitive and behavioral difficulties following the diagnosis and treatment of a brain tumor. Surgery, chemotherapy and radiation therapy have all been shown to impact the developing brain, especially the white matter.
Objective
The purpose of this study was to determine the long-term effects of radiation therapy on white matter integrity, as measured by diffusion tensor imaging, in pediatric brain tumor patients 2 years after the end of radiation treatment, while controlling for surgical interventions.
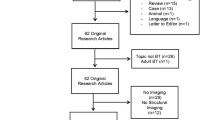
Materials and methods
We evaluated diffusion tensor imaging performed at two time points: a baseline 3 to 12 months after surgery and a follow-up approximately 2 years later in pediatric brain tumor patients. A region of interest analysis was performed within three regions of the corpus callosum. Diffusion tensor metrics were determined for participants (n=22) who underwent surgical tumor resection and radiation therapy and demographically matched with participants (n=22) who received surgical tumor resection only.
Results
Analysis revealed that 2 years after treatment, the radiation treated group exhibited significantly lower fractional anisotropy and significantly higher radial diffusivity within the body of the corpus callosum compared to the group that did not receive radiation.
Conclusion
The findings indicate that pediatric brain tumor patients treated with radiation therapy may be at greater risk of experiencing long-term damage to the body of the corpus callosum than those treated with surgery alone.

Similar content being viewed by others
References
Patel S, Bhatnagar A, Wear C et al (2014) Are pediatric brain tumors on the rise in the USA? Significant incidence and survival findings from the SEER database analysis. Childs Nerv Syst 30:147–154
Ostrom QT, Gittleman H, Fulop J et al (2015) CBTRUS statistical report: primary brain and central nervous system tumors diagnosed in the united states in 2008-2012. Neuro Oncol 17(Suppl 4):iv1-iv62
Greene-Schloesser D, Moore E, Robbins ME (2013) Molecular pathways: radiation-induced cognitive impairment. Clin Cancer Res 19:2294–2300
Conklin HM, Ashford JM, Di Pinto M et al (2013) Computerized assessment of cognitive late effects among adolescent brain tumor survivors. J Neuro-Oncol 113:333–340
Lee YW, Cho HJ, Lee WH, Sonntag WE (2012) Whole brain radiation-induced cognitive impairment: pathophysiological mechanisms and therapeutic targets. Biomol Ther 20:357–370
Palmer SL, Armstrong C, Onar-Thomas A et al (2013) Processing speed, attention, and working memory after treatment for medulloblastoma: an international, prospective, and longitudinal study. J Clin Oncol 31:3494–3500
Mulhern RK, Palmer SL, Reddick WE et al (2001) Risks of young age for selected neurocognitive deficits in medulloblastoma are associated with white matter loss. J Clin Oncol 19:472–479
Mulhern RK, Merchant TE, Gajjar A et al (2004) Late neurocognitive sequelae in survivors of brain tumours in childhood. Lancet Oncol 5:399–408
Dietrich J, Monje M, Wefel J, Meyers C (2008) Clinical patterns and biological correlates of cognitive dysfunction associated with cancer therapy. Oncologist 13:1285–1295
Mulhern RK, Palmer SL, Merchant TE et al (2005) Neurocognitive consequences of risk-adapted therapy for childhood medulloblastoma. J Clin Oncol 23:5511–5519
Ris MD, Packer R, Goldwein J et al (2001) Intellectual outcome after reduced-dose radiation therapy plus adjuvant chemotherapy for medulloblastoma: a Children's cancer group study. J Clin Oncol 19:3470–3476
Price RE, Langford LA, Jackson EF et al (2001) Radiation-induced morphologic changes in the rhesus monkey (Macaca Mulatta) brain. J Med Primatol 30:81–87
Vogel FS, Hoak CG, Sloper JC, Haymaker W (1958) The induction of acute morphological changes in the central nervous system and pituitary body of macaque monkeys by cobalt60 (gamma) radiation. J Neuropathol Exp Neurol 17:138–150
Burns TC, Awad AJ, Li MD, Grant GA (2016) Radiation-induced brain injury: low-hanging fruit for neuroregeneration. Neurosurg Focus 40:E3
Ishii A, Dutta R, Wark GM et al (2009) Human myelin proteome and comparative analysis with mouse myelin. Proc Natl Acad Sci U S A 106:14605–14610
Panagiotakos G, Alshamy G, Chan B et al (2007) Long-term impact of radiation on the stem cell and oligodendrocyte precursors in the brain. PLoS One 2:e588
Schultheiss TE, Stephens LC (1992) The pathogenesis of radiation myelopathy: widening the circle. Int J Radiat Oncol Biol Phys 23:1089–1091 discussion 1093-1084
Nagesh V, Tsien CI, Chenevert TL et al (2008) Radiation-induced changes in normal-appearing white matter in patients with cerebral tumors: a diffusion tensor imaging study. Int J Radiat Oncol Biol Phys 70:1002–1010
Rueckriegel SM, Driever PH, Blankenburg F et al (2010) Differences in supratentorial damage of white matter in pediatric survivors of posterior fossa tumors with and without adjuvant treatment as detected by magnetic resonance diffusion tensor imaging. Int J Radiat Oncol Biol Phys 76:859–866
Chapman CH, Nagesh V, Sundgren PC et al (2012) Diffusion tensor imaging of normal-appearing white matter as biomarker for radiation-induced late delayed cognitive decline. Int J Radiat Oncol Biol Phys 82:2033–2040
Jiang H, van Zijl PC, Kim J et al (2006) DTIStudio: resource program for diffusion tensor computation and fiber bundle tracking. Comput Methods Prog Biomed 81:106–116
Systat Software I (2014) SigmaPlot for windows. Systat Software, Inc., San Jose
Soares JM, Marques P, Alves V, Sousa N (2013) A hitchhiker's guide to diffusion tensor imaging. Front Neurosci 7:31
Wozniak JR, Krach L, Ward E et al (2007) Neurocognitive and neuroimaging correlates of pediatric traumatic brain injury: a diffusion tensor imaging (DTI) study. Arch Clin Neuropsych 22:555–568
Le Bihan D, Mangin JF, Poupon C et al (2001) Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging 13:534–546
Basser PJ, Pierpaoli C (1996) Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 111:209–219
Basser PJ (1995) Inferring microstructural features and the physiological state of tissues from diffusion-weighted images. NMR Biomed 8:333–344
Basser PJ, Pajevic S, Pierpaoli C et al (2000) In vivo fiber tractography using DT-MRI data. Magn Reson Med 44:625–632
Xue R, van Zijl PC, Crain BJ et al (1999) In vivo three-dimensional reconstruction of rat brain axonal projections by diffusion tensor imaging. Magn Reson Med 42:1123–1127
Qiu D, Kwong DLW, Chan GCF et al (2007) Diffusion tensor magnetic resonance imaging finding of discrepant fractional anisotropy between the frontal and parietal lobes after whole-brain irradiation in childhood medulloblastoma survivors: reflection of regional white matter radiosensitivity? Int J Radiat Oncol Biol Phys 69:846–851
Khong PL, Kwong DLW, Chan GCF et al (2003) Diffusion-tensor imaging for the detection and quantification of treatment-induced white matter injury in children with medulloblastoma: a pilot study. AJNR Am J Neuroradiol 24:734–740
Uh J, Merchant TE, Li Y et al (2015) Effects of surgery and proton therapy on cerebral white matter of craniopharyngioma patients. Int J Radiat Oncol Biol Phys 93:64–71
Hope TR, Vardal J, Bjornerud A et al (2015) Serial diffusion tensor imaging for early detection of radiation-induced injuries to normal-appearing white matter in high-grade glioma patients. J Magn Reson Imaging 41:414–423
de Blank PM, Berman JI, Fisher MJ (2016) Systemic chemotherapy and white matter integrity in tracts associated with cognition among children with neurofibromatosis type 1. Pediatr Blood Cancer 63:818–824
Borghesani PR, Madhyastha TM, Aylward EH et al (2013) The association between higher order abilities, processing speed, and age are variably mediated by white matter integrity during typical aging. Neuropsychologia 51:1435–1444
Genova HM, DeLuca J, Chiaravalloti N, Wylie G (2013) The relationship between executive functioning, processing speed, and white matter integrity in multiple sclerosis. J Clin Exp Neuropsychol 35:631–641
Kerchner GA, Racine CA, Hale S et al (2012) Cognitive processing speed in older adults: relationship with white matter integrity. PLoS One 7:e50425
Kourtidou P, McCauley SR, Bigler ED et al (2013) Centrum semiovale and corpus callosum integrity in relation to information processing speed in patients with severe traumatic brain injury. J Head Trauma Rehabil 28:433–441
Ferrer E, Whitaker KJ, Steele JS et al (2013) White matter maturation supports the development of reasoning ability through its influence on processing speed. Dev Sci 16:941–951
Alexander AL, Hasan KM, Lazar M et al (2001) Analysis of partial volume effects in diffusion-tensor MRI. Magn Reson Med 45:770–780
Frank LR (2001) Anisotropy in high angular resolution diffusion-weighted MRI. Magn Reson Med 45:935–939
Oouchi H, Yamada K, Sakai K et al (2007) Diffusion anisotropy measurement of brain white matter is affected by voxel size: underestimation occurs in areas with crossing fibers. AJNR Am J Neuroradiol 28:1102–1106
Pfefferbaum A, Sullivan EV (2003) Increased brain white matter diffusivity in normal adult aging: relationship to anisotropy and partial voluming. Magn Reson Med 49:953–961
Yuan W, Mangano FT, Air EL et al (2009) Anisotropic diffusion properties in infants with hydrocephalus: a diffusion tensor imaging study. AJNR Am J Neuroradiol 30:1792–1798
Patel SK, Yuan W, Mangano FT (2017) Advanced neuroimaging techniques in pediatric hydrocephalus. Pediatr Neurosurg. doi:10.1159/000454717
Siasios I, Kapsalaki EZ, Fountas KN et al (2016) The role of diffusion tensor imaging and fractional anisotropy in the evaluation of patients with idiopathic normal pressure hydrocephalus: a literature review. Neurosurg Focus 41:E12
Acknowledgements
Funding to support this work came from the National Institutes of Health grant numbers R01 CA112182, R01 ES027724 and the Intellectual & Development Disabilities Research Center, at Kennedy Krieger Institute, grant number U54 HD079123.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest
None
Rights and permissions
About this article
Cite this article
Makola, M., Douglas Ris, M., Mahone, E.M. et al. Long-term effects of radiation therapy on white matter of the corpus callosum: a diffusion tensor imaging study in children. Pediatr Radiol 47, 1809–1816 (2017). https://doi.org/10.1007/s00247-017-3955-1
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-017-3955-1