Abstract
Background
Because of the ability of blood-oxygen-level-dependent (BOLD) MRI to assess blood oxygenation changes within the microvasculature, this technique holds potential for evaluating early perisynovial changes in inflammatory arthritis.
Objective
To evaluate the feasibility of BOLD MRI to detect interval perisynovial changes in knees of rabbits with inflammatory arthritis.
Materials and methods
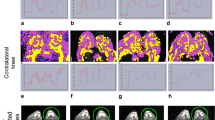
Rabbit knees were injected with albumin (n = 9) or saline (n = 6) intra-articularly, or were not injected (control knees, n = 9). Except for two rabbits (albumin-injected, n = 2 knees; saline-injected, n = 2 knees) that unexpectedly died on days 7 and 21 of the experiment, respectively, all other animals were scanned with BOLD MRI on days 0, 1, 7, 14, 21 and 28 after induction of arthritis. T2*-weighted gradient-echo MRI was performed during alternate 30 s of normoxia/hyperoxia. BOLD MRI measurements were compared with clinical, laboratory and histological markers.
Results
Percentage of activated voxels was significantly greater in albumin-injected knees than in contralateral saline-injected knees (P = 0.04). For albumin-injected knees (P < 0.05) and among different categories of knees (P = 0.009), the percentage of activated BOLD voxels varied over time. A quadratic curve for on-and-off BOLD difference was delineated for albumin- and saline-injected knees over time (albumin-injected, P = 0.047; saline-injected, P = 0.009). A trend toward a significant difference in synovial histological scores between albumin-injected and saline-injected knees was noted only for acute scores (P = 0.07).
Conclusion
As a proof of concept, BOLD MRI can depict perisynovial changes during progression of experimental arthritis.





Similar content being viewed by others
References
Stevens CR, Blake DR, Merry P, et al (1991) A comparative study by morphometry of the microvasculature in normal and rheumatoid synovium. Arthritis Rheum 34:1508–1513
Edmonds SE, Blake DR, Morris CJ, et al (1993) An imaginative approach to synovitis—the role of hypoxic reperfusion damage in arthritis. J Rheumatol Suppl 37:26–31
Padhani AR (2002) Dynamic contrast-enhanced MRI in clinical oncology: current status and future directions. J Magn Reson Imaging 16:407–422
Noseworthy MD, Bulte DP, Alfonsi J (2003) BOLD magnetic resonance imaging of skeletal muscle. Semin Musculoskelet Radiol 7:307–315
Novack P, Shenkin H, Bortin L, et al (1953) The effects of carbon dioxide inhalation upon the cerebral blood flow and cerebral oxygen consumption in vascular disease. J Clin Invest 32:696–702
Sicard KM, Duong TQ (2005) Effects of hypoxia, hyperoxia, and hypercapnia on baseline and stimulus-evoked BOLD, CBF, and CMRO2 in spontaneously breathing animals. Neuroimage 25:850–858
Prisman E, Slessarev M, Han J, et al (2008) Comparison of the effect of independently controlled end-tidal pCO2 and pO2 on blood oxygen level-dependent (BOLD) MRI. J Magn Reson Imag 27:185–191
Imrie RC (1976) Animal models of arthritis. Lab Anim Sci 26:345–351
Cooke TDV (1988) Antigen-induced arthritis, polyarthritis, and tenosynovitis. In: Greenwald RA, Diamond HS (eds) CRC handbook of animal models for rheumatoid diseases. CRC Press, Boca Raton, pp 53–79
Strouse PJ, DiPietro MA, Teo EL, et al (1999) Power Doppler evaluation of joint effusions: investigation in a rabbit model. Pediatr Radiol 29:617–623
Strouse PJ, Londy F, DiPietro MA, et al (1999) MRI evaluation of infectious and non-infectious synovitis: preliminary studies in a rabbit model. Pediatr Radiol 29:367–371
Glover GH, Lai S (1998) Self-navigated spiral fMRI: interleaved versus single-shot. Magn Reson Med 39:361–368
Haller S, Wetzel SG, Radue EW, et al (2006) Mapping continuous neuronal activation without an ON-OFF paradigm: initial results of BOLD ceiling fMRI. Eur J Neurosci 24:2672–2678
Doria AS, Dick PT (2005) Region-of-interest-based analysis of clustered BOLD MRI data in experimental arthritis. Acad Radiol 12:841–852
Frahm J, Merboldt KD, Hanicke W (1993) Functional MRI of human brain activation at high spatial resolution. Magn Reson Med 29:139–144
Field AS, Yen YF, Burdette JH, et al (2000) False cerebral activation on BOLD functional MR images: study of low-amplitude motion weakly correlated to stimulus. AJNR 21:1388–1396
Resnick D (1988) Common disorders of synovium-lined joints: pathogenesis, imaging abnormalities, and complications. AJR 151:1079–1093
Hunneyball IM, Spowage M, Crossley MJ, et al (1986) Acute phase protein changes in antigen-induced mono-articular arthritis in rabbits and mice. Clin Exp Immunol 65:311–318
Kane D, Roth J, Frosch M, et al (2003) Increased perivascular synovial membrane expression of myeloid-related proteins in psoriatic arthritis. Arthritis Rheum 48:1676–1685
Roth J, Teigelkamp S, Wilke M, et al (1992) Complex pattern of the myelo-monocytic differentiation antigens MRP8 and MRP14 during chronic airway inflammation. Immunobiology 186:304–314
Koizumi F, Matsuno H, Wakaki K, et al (1999) Synovitis in rheumatoid arthritis: scoring of characteristic histopathological features. Pathol Int 49:298–304
Oehler S, Neureiter D, Meyer-Scholten C, et al (2002) Subtyping of osteoarthritic synoviopathy. Clin Exp Rheumatol 20:633–640
Kim HK, Kerr RG, Cruz TF, et al (1995) Effects of continuous passive motion and immobilization on synovitis and cartilage degradation in antigen induced arthritis. J Rheumatol 22:1714–1721
Fleiss JL (1981) Statistical methods for rates and proportions. Wiley, New York
Altman DG (1991) Practical statistics for medical research. Chapman and Hall, London, pp 404–408
Svalastoga E, Kiaer T, Gronlund J (1989) Improved method to estimate oxygen consumption, diffusing capacity and blood flow of synovial membrane. Acta Vet Scand 30:113–119
James MJ, Cleland LG, Rofe AM, et al (1990) Intraarticular pressure and the relationship between synovial perfusion and metabolic demand. J Rheumatol 17:521–527
Simkin PA (1979) Synovial permeability in rheumatoid arthritis. Arthritis Rheum 22:689–696
Villringer A (2000) Physiologic changes during brain activation. In: Baert AL (ed) Functional MRI. Springer, Berlin, pp 1–13
Boxerman JL, Bandettini PA, Kwong KK, et al (1995) The intravascular contribution to fMRI signal change: Monte Carlo modeling and diffusion-weighted studies in vivo. Magn Reson Med 34:4–10
Mazurchuk R, Zhou R, Straubinger RM, et al (1999) Functional magnetic resonance (fMR) imaging of a rat brain tumor model: implications for evaluation of tumor microvasculature and therapeutic response. Magn Reson Imaging 17:537–548
Gati JS, Menon RS, Ugurbil K, et al (1997) Experimental determination of the BOLD field strength dependence in vessels and tissue. Magn Reson Med 38:296–302
Ugurbil K, Hu X, Chen W, et al (1999) Functional mapping in the human brain using high magnetic fields. Philos Trans R Soc Lond B Biol Sci 354:1195–1213
Yang Y, Wen H, Mattay VS, et al (1999) Comparison of 3D BOLD functional MRI with spiral acquisition at 1.5 and 4.0 T. Neuroimage 9:446–451
Robinson SP, Rodrigues LM, Griffiths JR, et al (1999) Response of hepatoma 9618a and normal liver to host carbogen and carbon monoxide breathing. Neoplasia 1:537–543
Storgard CM, Stupack DG, Jonczyk A, et al (1999) Decreased angiogenesis and arthritic disease in rabbits treated with an alphavbeta3 antagonist. J Clin Invest 103:47–54
Baltz ML, Gomer K, Davies AJ, et al (1980) Differences in the acute phase responses of serum amyloid P-component (SAP) and C3 to injections of casein or bovine serum albumin in amyloid-susceptible and -resistant mouse strains. Clin Exp Immunol 39:355–360
Brasch RC, Li KC, Husband JE, et al (2000) In vivo monitoring of tumor angiogenesis with MR imaging. Acad Radiol 7:812–823
Lee SP, Silva AC, Ugurbil K, et al (1999) Diffusion-weighted spin-echo fMRI at 9.4 T: microvascular/tissue contribution to BOLD signal changes. Magn Reson Med 42:919–928
Van Dijke CF, Peterfy CG, Brasch RC, et al (1999) MR imaging of the arthritic rabbit knee joint using albumin-(Gd-DTPA)30 with correlation to histopathology. Magn Reson Imaging 17:237–245
Shockley RP, LaManna JC (1988) Determination of rat cerebral cortical blood volume changes by capillary mean transit time analysis during hypoxia, hypercapnia and hyperventilation. Brain Res 454:170–178
Acknowledgements
The authors thank Michael Noseworthy for setting up the MR sequences used in this study and for reviewing the first draft of the manuscript, Sharon Nancekivell for editorial assistance, Dr. Brian Feldman for advice about the design of the study, Dr. Geraldine Kent for help with the preparation of the albumin solution, Marianne Rogers for histological services, Jeff Alfonsi and Niels Celeghin for analysis of data, Man Khun Chan and Dr. Adele Khoskow for suggesting and testing laboratory surrogates for inflammation in rabbits, and Hillary Bruce and Mary Ancona for collaborating as MR imaging technologists in part of the study.
Author information
Authors and Affiliations
Corresponding author
Additional information
In memoriam of Robert B. Salter.
Rights and permissions
About this article
Cite this article
Doria, A.S., Crawley, A., Gahunia, H. et al. Correlative BOLD MR imaging of stages of synovitis in a rabbit model of antigen-induced arthritis. Pediatr Radiol 42, 63–75 (2012). https://doi.org/10.1007/s00247-011-2194-0
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00247-011-2194-0