Abstract
Near infrared spectroscopy is routinely used in the noninvasive monitoring of cerebral and somatic regional oxygen saturation (rSO2) in pediatric patients following surgery for congenital heart disease. We sought to evaluate the association of a bedside rSO2 thought algorithm with clinical outcomes in a cohort of pediatric patients following cardiac surgery. This was a single-center retrospective cohort study of patients admitted following cardiac surgery over a 42-month period. The intervention was the implementation of an rSO2 thought algorithm, the primary goal of which was to supply bedside providers with a thought aide to help identify, and guide response to, changes in rSO2 in post-operative cardiac surgical patients. Surgical cases were stratified into two 18-month periods of observation, pre- and post-intervention allowing for a 6-month washout period during implementation of the thought algorithm. Clinical outcomes were compared between pre- and post-intervention periods. There were 434 surgical cases during the period of study. We observed a 27% relative risk reduction in our standardized mortality rate (0.61 to 0.48, p = 0.01) between the pre- and post-intervention periods. We did not observe differences in other post-operative clinical outcomes such as ventilator free days or post-operative ICU length of stay. Providing frontline clinical staff with education and tools, such as a bedside rSO2 thought algorithm, may aide in the earlier detection of imbalance between oxygen delivery and consumption and may contribute to improved patient outcomes.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Near infrared spectroscopy (NIRS) is routinely used in the noninvasive monitoring of cerebral and somatic regional oxygen saturation (rSO2) in pediatric patients following surgery for congenital heart disease [1,2,3]. Monitoring of cerebral and somatic rSO2 can provide clinicians with insights into regional oxygen delivery and consumption following cardiac surgery [3,4,5,6,7,8]. Multiple studies have demonstrated associations between rSO2 values obtained following cardiac surgery in pediatric patients and postoperative outcomes such as mortality, ventilator free days, and low cardiac output syndrome [9,10,11,12].
For many practitioners, cerebral and somatic rSO2 are viewed as routinely monitored vital signs not unlike mean arterial pressure or arterial oxygen saturation in postoperative cardiac surgical patients. While clear target ranges and treatment approaches to abnormal values are established for several monitored values (e.g., mean arterial pressure) following cardiac surgery, this practice has not fully been extended to rSO2 monitoring [13]. We sought to evaluate the association of a bedside rSO2 thought algorithm with clinical outcomes in a cohort of pediatric patients following cardiac surgery.
Materials and Methods
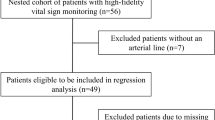
We conducted a retrospective cohort study of patients admitted following cardiac surgery to the pediatric intensive care unit (PICU) between October 1, 2017, and March 31, 2021, at the University of Virginia Children’s Hospital, an academic, tertiary-care center. The Institutional Review Board for Human Subjects Research at the University of Virginia School of Medicine (Protocol #19035) approved this study. We followed the Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) guidelines [14].
We included patients less than or equal to 18 years of age that underwent cardiac surgery with cardiopulmonary bypass (CPB). Additionally, we included patients that underwent cardiac surgery without CPB for the following procedures: pulmonary artery banding, systemic to pulmonary shunting (e.g., Blalock-Taussig-Thomas shunt), and coarctation of the aorta repair.
Multiple surgeries in an individual patient over the course of the study were considered independent surgical cases if they occurred during separate hospitalizations. In the operating room, cerebral and somatic oximetry sensors were placed on the forehead and right flank below the costovertebral angle overlying the right kidney, respectively. Values of cerebral and somatic rSO2 were continuously monitored in the PICU using the INVOS™ oximeter (Medtronic, Minneapolis, MN). Duration of post-operative rSO2 monitoring was at the discretion of the clinical team.
A multidisciplinary group of PICU providers developed the thought algorithm (Supplementary Fig. 1) through consensus agreement. The primary goal of the implementation was to supply bedside providers with a thought aide to help identify, and guide response to, changes in rSO2 in post-operative cardiac surgical patients. The primary bedside nurse was instructed to complete the thought algorithm upon admission to the PICU following cardiac surgery and after initiation stabilization and resuscitation of the patient within the first hour following admission from the Operating Room. Completion of the thought algorithm included establishing baseline cerebral and somatic rSO2 values based on the individual patient’s physiology as well as thresholds that might suggest significant changes in oxygen delivery or oxygen consumption. Orientation to the thought algorithm as well as background education on rSO2 monitoring was provided to PICU staff (nurses, house staff, respiratory therapists, advanced practice providers, and attending physicians) through a variety of mechanisms (e.g., online education modules, in-person lectures) over a two-month period prior to implementation in June 2019. Our primary objective was to assess differences in clinical outcomes over two 18-month periods before and after thought algorithm implementation. We designated the pre-intervention period as October 2017 to March 2019, and the post-intervention period as October 2019 to March 2021, allowing for a six-month washout period from April 2019 to September 2019. During the period of study, there were no changes in the cardiac surgical or pediatric critical care faculty or in the provision of post-operative care of patients following cardiac surgery.
We collected patient and clinical characteristics including age at time of surgery, CPB and cross clamp times, cardiac diagnosis, surgical procedure, delayed chest closure, use of extracorporeal life support, and postoperative ventilator free days (VFD) and PICU length of stay. Postoperative VFDs was defined as the number of days of invasive mechanical ventilation following surgery to postoperative Day 28, with patients who died before Day 28 assigned zero. For the first 48 h following surgery, peak lactate was collected, and peak vasoactive inotropic score was calculated [15]. The Society of Thoracic Surgeons—European Association for Cardiothoracic Surgery Heart Surgery (STAT) mortality categories were assigned to each surgical procedure [16]. Standardized mortality ratios were calculated for the pre-intervention and post-intervention periods using the Society of Thoracic Surgeon Congenital Heart Surgery Database Mortality Risk Model [17].
We performed a sensitivity analysis on the subset of neonates (< 30 days of age) that underwent cardiac surgery with cardiopulmonary bypass to compare several NIRS-derived measures between time periods. Values of cerebral and somatic rSO2 (crSO2 and srSO2, respectively) were continuously captured using the INVOS™ oximeter over the first 48 h following surgery and averaged over 1-min intervals. In addition to average crSO2 and srSO2, we calculated crSO2 and srSO2 desaturation indices defined as the duration of time over the first 48 h following surgery that crSO2 and srSO2 were below 50% [12]. Variability of crSO2 and srSO2 were calculated using the root mean of successive squared differences [18].
Distribution of continuous variables was assessed using the Wilk-Shapiro test for normality. Continuous variables were compared using Student’s T test, Wilcoxon rank sum testing, or linear regression as appropriate. Categorical variables were compared using chi-square test or Fisher’s exact testing as appropriate. Multivariable regression analysis was performed to adjust for confounders. Type I error was set at 0.05. All calculations were performed using STATA/IC 12.1 (STATA Corporation, College Station, TX).
Results
There were 434 surgical cases included in the study with a median patient age at the time of surgery of 3 months (interquartile range 9 days–18 months). The demographic and clinical characteristics of the included patients are listed in Table 1. The distribution of surgical cases over the three time periods was: pre-intervention: 218 cases (50%); wash-out: 91 cases (21%); and post-intervention: 125 cases (29%).
Patient and clinical characteristics were compared between the pre-intervention and post-intervention periods (Table 2). Patients in the post-intervention period were younger at the time of surgery than patients in the pre-intervention period (median 1 month vs 3 months, p = 0.03). Patients in the post-intervention period were more likely to have delayed sternal closure (20% vs 11%, p = 0.03). We did not observe differences in peak lactate or VIS in the first 48 h following surgery, VFDs, or post-operative PICU length of stay between the study periods.
The observed mortality rate was identical for both study periods (3.2%). Delayed sternal closure, use of extracorporeal life support and peak lactate were all associated with observed mortality (p = 0.003, p < 0.001, and p = 0.025, respectively). Expected mortality was increased in the post-intervention period (p = 0.012). Controlling for significant confounders, study period, delayed sternal closure and peak lactate were all associated with expected mortality (p = 0.037, p < 0.001, and p = 0.009, respectively). The standardized mortality ratio for the pre-intervention period was 0.61 (95% CI 0.16–1.06) and for the post-intervention period was 0.48 (95% CI 0.01–0.95) (p = 0.01). This difference represents a 27% relative risk reduction in standardized mortality in the post-intervention period.
We included 125 neonates (68 pre-intervention, 57 post-intervention) in the NIRS-based measures sensitivity analysis. We did not observe differences in average crSO2, crSO2 or srSO2 variability, or crSO2 or srSO2 desaturation indices. Adjusting for single ventricle physiology, neonates in the post-intervention period had increased average srSO2 values over the first 48 h following surgery (p = 0.045) as compared to the pre-intervention period. Among neonates with single ventricle physiology, the average srSO2 value increased from 70 to 75% following the intervention.
Discussion
Following the implementation of a bedside rSO2 thought algorithm for pediatric patients following cardiac surgery, we observed a 27% decrease in our standardized mortality rate. We did not observe differences in other post-operative clinical outcomes such as peak VIS or lactate, ventilator free days, or post-operative ICU length of stay. In the cohort of neonates with single ventricle physiology, we did observe an increase in the average srSO2 value following the intervention.
While there are several studies demonstrating the association of rSO2 measures with both invasively measured parameters (e.g., central venous oxygen saturation) and clinical states (e.g., low cardiac output syndrome), there is a paucity of data to support the role rSO2 monitoring plays in impacting clinical outcomes [3,4,5,6, 10]. A recently published systematic review and meta-analysis by Hansen et al. included 25 randomized clinical trials involving 2606 adult and pediatric patients randomized to either cerebral rSO2 monitoring or no monitoring [19]. The Authors noted a high risk of bias in reported trials and concluded that the effects on clinical care with access to cerebral rSO2 monitoring versus clinical care without access to cerebral rSO2 monitoring remains uncertain [19].
From a practical standpoint, there are well-defined assessment and treatment algorithms for abnormalities in routine vital signs such as hypotension or hypoxemia. Inherent in this process is the care team’s ability to understand the physiologic property that is being measured by any given vital sign and possess a shared understanding of what that value represents. With rSO2 the interpretation of values may require a more nuanced approach. This characteristic may help explain why the use of NIRS monitoring is still viewed primarily as a trending tool by many practitioners [13]. Irrespective of how the end-user incorporates rSO2 measures into clinical decision making, NIRS is routinely used in the PICU following cardiac surgery, contributing to a lack of equipoise for the conduct of a randomized controlled trial in this population.
Our approach in this study was modeled on the work of Woods-Hill et al. who designed a novel framework to improve blood culture utilization in the PICU [20]. As one of the participating PICU’s in the Woods-Hill study, our team learned the importance of stakeholder involvement in the creation of the algorithm and practical education of the end-users which we integrated into our study approach [20]. We recognized that several processes would be necessary for successful adoption of the rSO2 thought algorithm, including understanding the physiology monitored by rSO2 and the science behind the thought algorithm and suggested clinical pathways [21]. Anecdotally, following our educational efforts and implementation of the thought algorithm, we observed increased attention to, and reporting of, rSO2 values by bedside providers in patients following cardiac surgery.
Our most interesting finding was the decrease in our standardized mortality ratio for the post-intervention period. In a cohort of healthy children who underwent acute normovolemic hemodilution, Fontana et al. demonstrated that significant drops in rSO2 occurred well before changes in lactate were observed [22]. We hypothesize that the monitoring of rSO2 and informed responses to changes to rSO2 contributed to the earlier detection of imbalance of oxygen homeostasis allowing for intervention and improved outcomes. While we did not observe a statistically significant difference in surgical complexity between periods, we did note a 21% increase in the rates of STAT 4 and STAT 5 cases in the post-intervention period which may contribute to the lack of differences observed in post-operative ventilator free days and post-operative PICU length of stay.
We did observe that patients in the post-intervention period were younger and more likely to have delayed sternal closure as compared to the pre-intervention period. We surmise that these differences were highly influenced by the COVID-19 pandemic, the early stages of which occurred during the post-intervention period and was characterized by routine postponement of non-urgent and elective cardiac surgical procedures. Additionally, the pandemic effect likely drove the increase in expected mortality in the post-intervention period. While we did not see an increase in observed mortality with the increase in expected mortality, caution is warranted when evaluating the impact of our intervention on mortality outcomes.
Our study has several limitations, most notably the single-center design and the semi-passive nature of our intervention. While end-users were provided with education on both the principles of rSO2 monitoring and the thought algorithm, we did not mandate or protocolize clinical responses to changes in rSO2 values nor did we attempt to assess knowledge retention over time. It should be acknowledged that our intervention may simply have led to an overall increased level of clinical engagement at the bedside in the post-intervention period, irrespective of rSO2 monitoring. A potential next step would be to randomize patients to a protocolized response versus standard of care. Randomizing to rSO2 monitoring or no monitoring seems impractical in the current practice environment for children following cardiac surgery.
Monitoring of rSO2 in children following cardiac surgery has become routine. Providing frontline clinical staff with education and tools, such as a bedside rSO2 thought algorithm, may aide in the earlier detection of imbalance between oxygen delivery and consumption and may contribute to improved patient outcomes. Future studies aimed at evaluating specific therapeutic response strategies are needed.
References
Ghanayem NS, Hoffman GM (2016) Near infrared spectroscopy as a hemodynamic monitor in critical illness. Pediatr Crit Care Med 17(8 Suppl 1):S201-206
Hoffman GM, Ghanayem NS, Tweddell JS (2005) Noninvasive assessment of cardiac output. Semin Thorac Cardiovasc Surg Pediatr Card Surg Annu 8:12–21
Zaleski KL, Kussman BD (2020) Near-infrared spectroscopy in pediatric congenital heart disease. J Cardiothorac Vasc Anesth 34(2):489–500
Li J, Van Arsdell GS, Zhang G, Cai S, Humpl T, Caldarone CA, Holtby H, Redington AN (2006) Assessment of the relationship between cerebral and splanchnic oxygen saturations measured by near-infrared spectroscopy and direct measurements of systemic haemodynamic variables and oxygen transport after the Norwood procedure. Heart 92(11):1678–1685
McQuillen PS, Nishimoto MS, Bottrell CL, Fineman LD, Hamrick SE, Glidden DV, Azakie A, Adatia I, Miller SP (2007) Regional and central venous oxygen saturation monitoring following pediatric cardiac surgery: concordance and association with clinical variables. Pediatr Crit Care Med 8(2):154–160
Chakravarti SB, Mittnacht AJ, Katz JC, Nguyen K, Joashi U, Srivastava S (2009) Multisite near-infrared spectroscopy predicts elevated blood lactate level in children after cardiac surgery. J Cardiothorac Vasc Anesth 23(5):663–667
Nagdyman N, Ewert P, Peters B, Miera O, Fleck T, Berger F (2008) Comparison of different near-infrared spectroscopic cerebral oxygenation indices with central venous and jugular venous oxygenation saturation in children. Paediatr Anaesth 18(2):160–166
Nagdyman N, Fleck T, Barth S, Abdul-Khaliq H, Stiller B, Ewert P, Huebler M, Kuppe H, Lange PE (2004) Relation of cerebral tissue oxygenation index to central venous oxygen saturation in children. Intensive Care Med 30(3):468–471
Hoffman GM, Ghanayem NS, Scott JP, Tweddell JS, Mitchell ME, Mussatto KA (2017) Postoperative cerebral and somatic near-infrared spectroscopy saturations and outcome in hypoplastic left heart syndrome. Ann Thorac Surg 103(5):1527–1535
Hickok RL, Spaeder MC, Berger JT, Schuette JJ, Klugman D (2016) Postoperative abdominal NIRS values predict low cardiac output syndrome in neonates. World J Pediatr Congenit Heart Surg 7(2):180–184
Spaeder MC, Klugman D, Skurow-Todd K, Glass P, Jonas RA, Donofrio MT (2017) Perioperative near-infrared spectroscopy monitoring in neonates with congenital heart disease: relationship of cerebral tissue oxygenation index variability with neurodevelopmental outcome. Pediatr Crit Care Med 18(3):213–218
Spaeder MC, Surma VJ (2021) Association of somatic regional oxygen saturation with clinical outcomes in neonates following cardiac surgery. Pediatr Crit Care Med 22(7):e415–e416
Hoskote AU, Tume LN, Trieschmann U, Menzel C, Cogo P, Brown KL, Broadhead MW (2016) A cross-sectional survey of near-infrared spectroscopy use in pediatric cardiac ICUs in the United Kingdom, Ireland, Italy, and Germany. Pediatr Crit Care Med 17(1):36–44
von Elm EAD, Egger M, Pocock SJ, Gøtzsche PC, Vandenbroucke JP (2007) STROBE Initiative: The Strengthening the Reporting of Observational Studies in Epidemiology (STROBE) statement: guidelines for reporting observational studies. Ann Intern Med 147:573–577
Gaies MG, Gurney JG, Yen AH, Napoli ML, Gajarski RJ, Ohye RG, Charpie JR, Hirsch JC (2010) Vasoactive-inotropic score as a predictor of morbidity and mortality in infants after cardiopulmonary bypass. Pediatr Crit Care Med 11(2):234–238
Surgery SoTS-EAfC (2020) Appendix C: The Society of Thoracic Surgeons - European Association for Cardio-Thoracic Surgery Congenital Heart Surgery Mortality Categories (STAT Mortality Categories)
O’Brien SM, Jacobs JP, Pasquali SK, Gaynor JW, Karamlou T, Welke KF, Filardo G, Han JM, Kim S, Shahian DM et al (2015) The society of thoracic surgeons congenital heart surgery database mortality risk model: part 1-statistical methodology. Ann Thorac Surg 100(3):1054–1062
Spaeder MC, Surma VJ (2021) Cerebral regional oxygen saturation variability in neonates following cardiac surgery. Pediatr Res 90(4):815–818
Hansen ML, Hyttel-Sorensen S, Jakobsen JC, Gluud C, Kooi EMW, Mintzer J, de Boode WP, Fumagalli M, Alarcon A, Alderliesten T et al (2022) Cerebral near-infrared spectroscopy monitoring (NIRS) in children and adults: a systematic review with meta-analysis. Pediatr Res. https://doi.org/10.1038/s41390-022-01995-z
Woods-Hill CZ, Lee L, Xie A, King AF, Voskertchian A, Klaus SA, Smith MM, Miller MR, Colantuoni EA, Fackler JC et al (2018) Dissemination of a novel framework to improve blood culture use in pediatric critical care. Pediatr Qual Saf 3(5):e112
Keim-Malpass J, Kitzmiller RR, Skeeles-Worley A, Lindberg C, Clark MT, Tai R, Calland JF, Sullivan K, Randall Moorman J, Anderson RA (2018) Advancing continuous predictive analytics monitoring: moving from implementation to clinical action in a learning health system. Crit Care Nurs Clin N Am 30(2):273–287
Fontana JLWL, Mongan PD, Sturm P, Martin G, Bünger R (1995) Oxygen consumption and cardiovascular function in children during profound intraoperative normovolemic hemodilution. Anesth Analg 80:219–225
Funding
None declared.
Author information
Authors and Affiliations
Contributions
MS conceived the study design, performed all data analyses and wrote the main manuscript text. MS, JK, EP, DW, CS and WH developed the thought algorithm. MS and JK led the thought algorithm education effort and clinical implementation. MS, CS and VS performed data collection and abstraction. All authors reviewed the manuscript.
Corresponding author
Ethics declarations
Conflict of interest
None declared.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
All work was performed at the University of Virginia Children’s Hospital and the University of Virginia School of Medicine, Charlottesville, VA.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
Springer Nature or its licensor (e.g. a society or other partner) holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Spaeder, M.C., Keller, J.M., Sawda, C.N. et al. Implementation of a Regional Oxygen Saturation Thought Algorithm and Association with Clinical Outcomes in Pediatric Patients Following Cardiac Surgery. Pediatr Cardiol 44, 940–945 (2023). https://doi.org/10.1007/s00246-022-03071-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-022-03071-z