Abstract
Children with COVID-19 usually show milder symptoms than adults; however, a minority of them may have cardiac involvement. We aimed to identify the role of troponin I levels that may predict early cardiac involvement in children with COVID-19. A single-center retrospective study was conducted to evaluate hospitalized children diagnosed with COVID-19 between March 11, 2020, and December 31, 2021. Patients with available troponin I levels and with no known cardiac disease were included. During the study period, 412 children with COVID-19 who had troponin I levels on admission were identified. Troponin levels were elevated in 7 (1.7%) patients and were normal in 395 (98.3%) patients. The median age of patients with elevated troponin levels was 4 (min. 2–max. 144) months, which was statistically lower than the age of patients with normal troponin levels (P = 0.035). All the patients with elevated troponin levels had tachycardia. Out of 7 patients with high troponin levels, 3 (42.9%) of them were admitted to the pediatric intensive care unit (PICU), 2 (28.6%) required oxygen support, and 1 (14.3%) required a mechanical ventilator. Patients with elevated troponin levels had a statistically longer hospital stay (P < 0.001). Neutropenia, tachycardia, PICU admission, oxygen support, and mechanical ventilation were statistically more common in patients with elevated troponin levels (P values were 0.033, 0.020, < 0.001, 0.050, and < 0.001, respectively). Electrocardiography (ECG) and echocardiography (ECHO) were performed on all patients with elevated troponin levels, and 6 (85.8%) patients were diagnosed with myocarditis. The ECG and ECHO have been performed in 58 (14.3%) out of 405 patients with normal troponin levels. Two (3.5%) patients had negative T waves on ECG, and all ECHOs were normal. Our results suggest that elevated troponin I levels in children with COVID-19 can be used to evaluate cardiac involvement and decide the need for further pediatric cardiologist evaluation.
Similar content being viewed by others
Introduction
Coronavirus disease 2019 (COVID-19) has caused a global pandemic resulting in more than 5.600.000 deaths worldwide [1]. The main clinical manifestation of COVID-19 is pneumonia; however, it can also cause extrapulmonary findings, including cardiovascular disorders [2]. Recent studies about the cardiac manifestations of COVID-19 primarily focused on adults and reported that myocardial injury is a common finding and associated with disease severity and mortality [2, 3]. Cardiac troponin value is a highly sensitive and specific marker of myocardial damage [4]. Several studies showed that elevated troponin was associated with mortality, and the early measurement of cardiac troponin can be useful for risk classification in adult patients with COVID-19 [2,3,4,5].
COVID-19 can be asymptomatic in children, and they usually have a milder disease than adults; thus, it was thought COVID-19 did not cause a significant health problem in children [6]. However, COVID-19 rarely can cause cardiac disorders in children during acute infection or the resulting multisystem inflammatory syndrome in children (MIS-C) [7]. There are limited studies in the literature on the use of troponin levels and the potential role in predicting the cardiac involvement of COVID-19 in children [8,9,10]. In addition, when to have a consultation for a cardiology specialist is not clear, and the previous studies reported an increased workload of the pediatric cardiology department [11,12,13].
Despite the adult studies, the role of troponin levels on myocardial function is not well established in children with COVID-19. Therefore, we aimed to evaluate the demographic, clinical, and laboratory findings of COVID-19 in children and determine the role of troponin I levels in predicting cardiac involvement and prognosis, in addition to the impact on the decision of cardiology consultation.
Materials and Methods
Study Design and Study Population
A single-center retrospective study was conducted between March 11, 2020, and December 31, 2021, at Dr. Behcet Uz Child Disease and Pediatric Surgery Training and Research Hospital. It is a referral center for pediatric patients in the Aegean region of Turkey. COVID-19 was diagnosed according to the Turkey Ministry of Health COVID-19 Guideline and confirmed cases defined as the cases in which SARS-CoV-2 was detected by molecular methods from nasopharyngeal and oropharyngeal swab specimens [14].
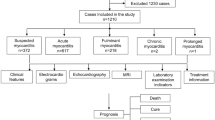
All hospitalized patients aged between 1 month and 18 year old with a positive SARS-CoV-2 real-time reverse transcriptional polymerase chain reaction (RT-PCR) were evaluated. The patients were retrospectively identified through the medical records. Children with a known specific cardiac disease, diagnosed as MIS-C, and who had any available troponin I level on admission were excluded. The scheme of the study design is shown in Fig. 1. A clinical scoring system was used to identify clinical classifications of illness severity in confirmed COVID-19 patients [15]. Tachycardia was defined as the presence of a heart rate value higher than expected for age [16].
In our center, a three-tiered clinical classification was used for the clinical diagnosis of myocarditis in children [17]. And also, we used revised Lake Louis criteria for patients whose cardiac magnetic resonance imaging (MRI) could be performed to diagnose myocarditis [18].
Data Collection
A standardized form was used to collect epidemiological data, laboratory findings, and patient's clinical symptoms and illness severity. Laboratory analysis on admission, including cardiac troponin I, white blood cell (WBC), absolute neutrophil count (ANC), absolute lymphocyte count (ALC), hemoglobin (Hb), platelet count (PLT), and C-reactive protein (CRP), values were also recorded. Thrombocytopenia was defined as a blood platelet count < 150 × 109/L, neutropenia was defined as absolute neutrophil count < 1.5 × 109/L, and lymphopenia was defined as absolute lymphocyte count < 1.5 × 109/L. Elevated CRP levels defined as CRP > 0.5 mg/dL.
The normal range of troponin I levels in our center is 002–0.06 ng/mL. A pediatric cardiologist evaluated all patients with elevated troponin I levels, and electrocardiography (ECG) and transthoracic echocardiography (ECHO) were performed. The patients were classified into two groups according to troponin levels: elevated troponin I levels and normal troponin I levels.
Statistical Analysis
The descriptive statistical analysis was performed using SPSS statistical software (version 22; SPSS, Chicago, IL, USA). Data were expressed as means ± standard deviation (SD) or medians (interquartile range) for continuous variables or percentages for categorical variables. The categorical variables are expressed as number (n) and percentage (%). The Student’s t test was used to compare continuous parametric variables, the Mann–Whitney U test was used to compare continuous nonparametric variables, and categorical variables were compared using Pearson χ2 or Fisher’s exact tests. A P value ≤ 0.05 was considered statistically significant.
This study was approved by the Local Ethical Committee of Dr. Behcet Uz Children's Training and Research Hospital (Decision No: 2022105).
Results
The study groups consisted of 412 patients with confirmed COVID-19 cases. The median age was 72 months (1–214 months), and 51% (n = 210) were male. Three hundred twenty-three (78.4%) patients were previously healthy, and the most common underlying disease in COVID-19 patients was obesity (n = 26, %29.2), followed by hematological-oncological malignancy (n = 15, 16.8%) and neurologic disease (n = 10, 11.2%). When patients were classified according to the clinical severity of the disease, 47 (11.4%) patients were asymptomatic, 309 (75%) mild, 40 (9.7%) moderate, 12 (2.9%) severe, and 4 (1%) critically ill in COVID-19 patients. Out of 412 patients, 14 (3.4%) patients required oxygen support, and 3 (0.7%) patients' respiratory support. No patient died, but 13 (3.2%) patients were admitted to the pediatric intensive care unit (PICU). Table 1 summarizes the demographic and clinical characteristics of the study population.
Among the hospitalized patients with COVID-19, 108 (26.2%) had neutropenia, 83 (20.1%) had lymphopenia, 40 (9.7%) had thrombocytopenia, and 145 (35.7%) had elevated CRP levels. Table 2 summarizes the laboratory characteristics of the study population.
Troponin I levels were elevated in 7 (1.7%) patients and were in the normal range in 405 patients (98.3%). The median age of patients with elevated troponin was 4 (min. 2–max. 144) months, and the median age of patients with normal troponin I levels was 84 (min. 1-max. 214) months. The median age of the patients with elevated troponin I levels was statistically lower than patients with normal troponin I levels (P < 0.035). When considering gender, no statistical difference among the groups was noted (P = 1.000). Three (42.9%) patients were mild, 2 (28.6%) were moderate, 1 (14.3%) was severe, and 1 (14.3%) was critical in the elevated troponin I levels group. In the normal troponin I levels group, 47 (11.6%) patients were asymptomatic, 306 (75.6%) were mild, 38 (9.4%) were moderate, 11 (2.7%) were severe, and 3 (0.7%) were critical. The rate of patients with an underlying disease was not different between the groups (P = 1.000). The length of hospital stay was found to be statistically longer (13 days, 4–56 days) in patients with elevated troponin I levels than patients with normal troponin I levels (4 days, 1–55 days) (P < 0.001).
All the patients with elevated troponin I levels had tachycardia. Out of 7 patients with elevated troponin levels, 3 (42.9%) of them were admitted to PICU, 2 (28.6%) required oxygen support, and 1 (14.3%) required mechanical ventilator support. Of the patients with normal troponin I levels, 94 (23.2%) had tachycardia, 10 (2.5%) of them were admitted to PICU, 12 (3%) required oxygen support, and 2 (0.5%) required mechanical ventilator support. Tachycardia, PICU admission, oxygen support, and mechanical ventilation requirement were statistically more common in the patients with elevated troponin I levels (P values were 0.020, < 0.001, 0.050, and < 0.001, respectively). Table 3 summarizes the demographic, clinical, and laboratory characteristics of the patient groups.
Among the patients, 79 (19.5%) patients had neutropenia, 108 (26.7%) had lymphopenia, 40 (9.9%) had thrombocytopenia, and 144 (35.6%) had elevated CRP levels in normal troponin I levels. However, in patients with elevated troponin I levels, 4 (57.1%) patients had neutropenia, 1 (14.3%) had elevated CRP levels, and no patients had lymphopenia or thrombocytopenia. The proportion of neutropenia as significantly higher in the patients with elevated troponin I levels (P = 0.033). The proportion of lymphopenia, thrombocytopenia, and high CRP level rates was not statistically different between the groups (P values were 0.197, 1.000, and 0.429, respectively). Table 3 summarizes the patient groups' demographic, clinical, and laboratory characteristics.
The ECG and ECHO were performed on all patients with elevated troponin I levels, and 6 (85.8%) were diagnosed with myocarditis. Table 4 summarizes the demographic and clinical characteristics of the patients diagnosed with myocarditis. The ECG and ECHO have been performed in 58 (14.3%) patients with normal troponin I levels. Two (3.5%) patients had negative T waves (in 1 patient, negative T waves in V4-V5 and the other patient in V1–V6) in ECG, and all of the ECHO were normal. Regarding the ECG and ECHO findings, no myocarditis involvement of COVID-19 infection was observed in the patient group with normal troponin I. Figure 2 shows the flow chart of troponin I levels of children hospitalized with COVID-19, and Fig. 3 shows the troponin I levels of children diagnosed with myocarditis.
Discussion
In this study, we evaluated 412 pediatric patients with COVID-19 and their troponin I levels. Troponin I levels were elevated in 7 (1.7%) patients, and six (85.8%) of them, the ECG and ECHO were compatible with myocarditis. In 405 (98.3%) patients, troponin I levels were normal, ECG and ECHO were performed 58 (14.3%) of them. All ECHOs were normal in patients with normal troponin I levels, and only 2 (3.5%) patients had abnormal ECGs. Our study suggests that elevated troponin I levels in children with COVID-19 are usually associated with myocarditis and should be evaluated by a pediatric cardiologist for further evaluation. In other words, in the patients with normal troponin I values without any hypotension and tachycardia, pediatric cardiology consultation is not required.
COVID-19 presents primarily with pulmonary disease; however, especially in adults, a significant portion of patients have cardiac involvement [2,3,4]. Like adults, children can have cardiac involvements such as arrhythmia, myocarditis, and cardiogenic shock with MIS-C associated with SARS-CoV-2 infection [7, 8]. The pathogenesis of the cardiac damage of COVID-19 is not clear in children. The reported causes of cardiac damage are increased cytokines and immune-inflammatory response, invasion of cardiomyocytes with SARS-CoV-2, lung failure, and hypoxia leading to oxidative stress and damage in cardiomyocytes [9, 19].
Acute myocarditis (AM) is defined as inflammation of the myocardium and can be a complication of acute viral infections [20]. Yoldas et al. [21] evaluated children with elevated troponin levels. They reported the most common pathologies in cardiac etiology were myopericarditis and perimyocarditis and can be diagnosed by history, physical examination, ECG, and ECHO. Vukomanovic et al. [20] evaluated 24 children with acute myocarditis. Seven of them were diagnosed with SARS-CoV-2 related acute myocarditis, and the others were unrelated to SARS-CoV-2. Patients with acute myocarditis related to SARS-CoV-2 had a higher CRP value and N‑terminal pro B‑type natriuretic peptide (NT-proBNP) levels; they had lower serum cardiac troponin I levels and platelets. In our study, 14.3% of the patients with elevated troponin levels also had elevated CRP levels.
In adult patients with COVID-19, increases in cardiac troponin levels indicate a myocardial injury and are associated with adverse outcomes such as arrhythmias and death [3]. Recent reports showed that the mortality rate was higher in patients with elevated cardiac troponin I levels in adults patients [22, 23]. There was no death in our study group with or without elevated troponin levels. The rate of PICU admission, oxygen support, and mechanical ventilation support requirement was higher in patients with elevated troponin I levels. Dweck et al. [24] reported that out of 1216 adult patients diagnosed with COVID-19, 55% of patients had an abnormal echocardiogram. They reported left and right ventricular abnormalities were 39% and 33%, respectively, with evidence of new myocardial infarction in 3%, myocarditis in 3%, and takotsubo cardiomyopathy in 2%.
Cardiac involvement associated with MIS-C has been commonly reported, but the characteristics of cardiac involvement in children with SARS-CoV-2 infection have not been well described. Fremed et al. [25] evaluated 80 pediatric patients and excluded MIS-C and chronic heart disease patients, like our study. They reported that high-sensitivity troponin T and/or NT-proBNP were measured in 34% of patients, and abnormalities were present in 19%, all of whom had underlying comorbidities. ECG abnormalities were identified in 37%, ECHO was performed on 7/80 patients, and none demonstrated left ventricular dysfunction. Respiratory support was required in all patients with elevated cardiac biomarkers. In our study, troponin I levels were elevated in 1.7% of patients, and 85.8% of the ECG and ECHO were compatible with myocarditis. In contrast to this report, only one patient with elevated troponin had an underlying disease and required mechanical ventilation. Ramoglu et al. [10] evaluated 214 pediatric patients, and troponin T levels were elevated in 15 (7%) patients. Troponin T positivity was significantly higher in patients under 12 months, and CRP levels were elevated in 46.7% of patients with troponin positivity. Similar to this study, in our study, the median age (4 months) of the patients with elevated troponin I levels was under 12 months and statistically younger than patients with normal troponin levels. Contrary to our study, they reported that ECHO evaluation of all patients with troponin positivity was normal, and there were no ECG changes except sinus tachycardia in one patient with fever.
Güllü et al. [8] evaluated 320 pediatric patients, and five were diagnosed with MIS-C. They reported that cardiac markers (NT-proBNP and troponin I), especially NT-proBNP, could be used to detect early diagnosis of cardiac involvement and/or MIS-C in pediatric patients with COVID-19. Cantarutti et al. [7] evaluated 294 children with active or previous SARS-COV-2 infection for the cardiac manifestation of SARS-COV-2 infection. Out of 248 patients without MIS-C, 2% had elevated troponin T levels, 18% had ECG anomalies, and 5% had ECHO anomalies. The rate of elevated troponin levels was similar to our study. During the COVID-19 pandemic, an increase in cardiologist consultation was reported in adults [13]. In children with COVID-19, a pediatric cardiologist consultation is frequently requested, and ECHO is performed due to concern for cardiac involvement. However, pediatric cardiologist consultation and ECHO performance can be reduced by using troponin I levels as a simple laboratory test.
This study had limitations, including its retrospective nature and lack of the advantages of randomized control studies. It was a single-center study, and limited numbers of patients were analyzed. In addition, we evaluated troponin levels at the time of admission, and they might be changed during the illness. In addition, we only included the patients who were evaluated with ECHO in the normal troponin I group in the final analysis. However, our study gives information about using troponin levels to determine the requirement of further cardiac evaluation by a pediatric cardiologist.
In conclusion, the analysis of troponin is a simple laboratory test and can provide important information on cardiac involvement. This study showed that elevated troponin levels with tachycardia are associated with myocarditis. We suggest that elevated troponin I levels in children with COVID-19 can be used to evaluate cardiac involvement and decide the need for further pediatric cardiologist evaluation. In other words, in the patients with normal troponin I values without any hypotension and tachycardia, pediatric cardiology consultation is not required.
References
World Health Organization (2022) WHO Coronavirus (COVID-19) Dashboard. https://covid19.who.int. Accessed 30 Jan 2022
García de Guadiana Romualdo L, Morell-García D, Rodríguez-Fraga O, Morales-Indiano C, María Lourdes Padilla Jiménez A, Gutiérrez Revilla JI et al (2021) Cardiac troponin and COVID-19 severity: results from BIOCOVID study. Eur J Clin Invest 51(6):e13532. https://doi.org/10.1111/eci.13532
Sandoval Y, Januzzi JL Jr, Jaffe AS (2020) Cardiac troponin for assessment of myocardial injury in COVID-19: JACC review topic of the week. J Am Coll Cardiol 76(10):1244–1258. https://doi.org/10.1016/j.jacc.2020.06.068
Imazio M, Klingel K, Kindermann I, Brucato A, De Rosa FG, Adler Y, De Ferrari GM (2020) COVID-19 pandemic and troponin: indirect myocardial injury, myocardial inflammation or myocarditis? Heart 106(15):1127–1131. https://doi.org/10.1136/heartjnl-2020-317186
Wibowo A, Pranata R, Akbar MR, Purnomowati A, Martha JW (2021) Prognostic performance of troponin in COVID-19: a diagnostic meta-analysis and meta-regression. Int J Infect Dis 105:312–318. https://doi.org/10.1016/j.ijid.2021.02.113
Ludvigsson JF (2020) Systematic review of COVID-19 in children shows milder cases and a better prognosis than adults. Acta Paediatr 109(6):1088–1095. https://doi.org/10.1111/apa.15270
Cantarutti N, Battista V, Adorisio R, Cicenia M, Campanello C, Listo E, Campana A, Trocchio G, Drago F (2021) Cardiac manifestations in children with SARS-COV-2 infection: 1-year pediatric multicenter experience. Children (Basel) 8(8):717. https://doi.org/10.3390/children8080717
Güllü UU, Güngör Ş, İpek S, Yurttutan S, Dilber C (2021) Predictive value of cardiac markers in the prognosis of COVID-19 in children. Am J Emerg Med 48:307–311. https://doi.org/10.1016/j.ajem.2021.06.075
Çevik BŞ, Arıcı Ş, Ergenç Z, Kepenekli E, Günal Ö, Yakut N (2021) How safe are children with COVID-19 from cardiac risks? Pediatric risk assesment; insights from echocardiography and electrocardiography. Turk J Med Sci 51(3):981–990. https://doi.org/10.3906/sag-2010-240
Ramoğlu MG, Karagözlü S, Bayram Ö, Bakhtiyarzada J, Aydın A, Vatansever G, Özdemir H, Tekin D, Uçar T, Çiftçi E, Tutar E (2022) The role and efficacy of routine high-sensitivity troponin T screening in paediatric COVID-19. Cardiol Young. https://doi.org/10.1017/S1047951121005199
Fremed MA, Niaz T, Hope KD, Altman CA, Levy VY, Glickstein JS, Johnson JN (2021) Adaptations of paediatric cardiology practice during the COVID-19 pandemic. Cardiol Young. https://doi.org/10.1017/S1047951121004364
Niaz T, Hope K, Fremed M, Misra N, Altman C, Glickstein J, Sanchez-de-Toledo J, Fraisse A, Miller J, Snyder C, Johnson JN, Chowdhury D (2021) Role of a pediatric cardiologist in the COVID-19 pandemic. Pediatr Cardiol 42(1):19–35. https://doi.org/10.1007/s00246-020-02476-y
Babat N, Duz R, Karaduman M, Tuncer M (2021) Evaluation of cardiology consultations during the COVID-19 pandemic period. East J Med 26(3):487–493. https://doi.org/10.5505/ejm.2021.02359
Turkey Ministry of Health, General Directorate of Public Health (2022) COVID-19 Guide (April 14, 2020). https://covid19bilgi.saglik.gov.tr/depo/rehberler/covid-19_rehberi.pdf. Accessed 30 Jan 2022
Carlotti APCP, Carvalho WB, Johnston C, Rodriguez IS, Delgado AF (2020) COVID-19 diagnostic and management protocol for pediatric patients. Clinics (Sao Paulo) 75:e1894. https://doi.org/10.6061/clinics/2020/e1894
Park MK, Guntheroth WG (2006) How to read pediatric ECGs, 4th edn. Mosby Elsevier, Philadelphia
Sagar S, Liu PP, Cooper LT Jr (2012) Myocarditis. Lancet 379(9817):738–747. https://doi.org/10.1016/S0140-6736(11)60648-X
Ferreira VM, Schulz-Menger J, Holmvang G, Kramer CM, Carbone I, Sechtem U, Kindermann I, Gutberlet M, Cooper LT, Liu P, Friedrich MG (2018) Cardiovascular magnetic resonance in nonischemic myocardial inflammation: expert recommendations. J Am Coll Cardiol 72(24):3158–3176. https://doi.org/10.1016/j.jacc.2018.09.072
Joshi K, Kaplan D, Bakar A, Jennings JF, Hayes DA, Mahajan S, Misra N, Mitchell E, Sweberg TM, Taylor MD, Capone CA (2020) Cardiac dysfunction and shock in pediatric patients with COVID-19. JACC Case Rep 2(9):1267–1270. https://doi.org/10.1016/j.jaccas.2020.05.082
Vukomanovic VA, Krasic S, Prijic S, Ninic S, Minic P, Petrovic G, Nesic D (2021) Differences between pediatric acute myocarditis related and unrelated to SARS-CoV-2. Pediatr Infect Dis J 40(5):e173–e178. https://doi.org/10.1097/INF.0000000000003094
Yoldaş T, Örün UA (2019) What is the significance of elevated troponin i in children and adolescents? A diagnostic approach. Pediatr Cardiol 40(8):1638–1644. https://doi.org/10.1007/s00246-019-02198-w
Ali J, Khan FR, Ullah R, Hassan Z, Khattak S, Lakhta G, Zad Gul N, Ullah R (2021) Cardiac troponin I levels in hospitalized COVID-19 patients as a predictor of severity and outcome: a retrospective cohort study. Cureus 13(3):e14061. https://doi.org/10.7759/cureus.14061
Salvatici M, Barbieri B, Cioffi SMG, Morenghi E, Leone FP, Maura F, Moriello G, Sandri MT (2020) Association between cardiac troponin I and mortality in patients with COVID-19. Biomarkers 25(8):634–640. https://doi.org/10.1080/1354750X.2020.1831609
Dweck MR, Bularga A, Hahn RT, Bing R, Lee KK, Chapman AR, White A, Salvo GD, Sade LE, Pearce K, Newby DE, Popescu BA, Donal E, Cosyns B, Edvardsen T, Mills NL, Haugaa K (2020) Global evaluation of echocardiography in patients with COVID-19. Eur Heart J Cardiovasc Imaging 21(9):949–958. https://doi.org/10.1093/ehjci/jeaa178
Fremed MA, Healy EW, Choi NH, Cheung EW, Choudhury TA, Jiang P, Liberman L, Zucker J, Lytrivi ID, Starc TJ (2022) Elevated cardiac biomarkers and outcomes in children and adolescents with acute COVID-19. Cardiol Young. https://doi.org/10.1017/S1047951122000397
Funding
The authors declare that no funds, grants, or other support were received during the preparation of this manuscript.
Author information
Authors and Affiliations
Contributions
All authors contributed to the study conception and design. All authors read and approved the final manuscript.
Corresponding author
Ethics declarations
Conflict of interest
The authors have no relevant financial or non-financial interests to disclose.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
Springer Nature or its licensor holds exclusive rights to this article under a publishing agreement with the author(s) or other rightsholder(s); author self-archiving of the accepted manuscript version of this article is solely governed by the terms of such publishing agreement and applicable law.
About this article
Cite this article
Guner Ozenen, G., Akaslan Kara, A., Kiymet, E. et al. The Evaluation of Troponin I Levels and Myocarditis in Children with COVID-19: A Pediatric Single-Center Experience. Pediatr Cardiol 44, 873–881 (2023). https://doi.org/10.1007/s00246-022-03017-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-022-03017-5