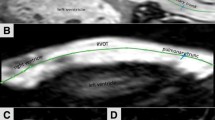
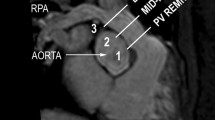
Abstract
Percutaneous pulmonary valve replacement (PPVI) in native or patched right ventricular outflow tract (RVOT) has proven to be feasible. The procedure is highly dependent on the size of the RVOT. Several methods exist to evaluate the size of the RVOT by cardiovascular magnetic resonance (CMR). We evaluated different CMR modalities for measuring RVOT diameters. Thirty-one consecutive patients with native or patched RVOT were retrospectively evaluated. CMR was part of follow-up of patients with corrected Tetralogy of Fallot or pulmonary stenosis with significant pulmonary regurgitation (PR). CMR included 3D-SSFP whole-heart in systole, diastole, and contrast-enhanced MR angiography (ceMRA). Diameters of the RVOT were assessed by the three sequences. Additionally, in patients who underwent cardiac catheterization (n = 11) for PPVI, vessel diameters assessed by cine-angiography were compared to CMR. Systolic diameters of RVOT were significantly larger compared to the diameters taken in diastole and ceMRA (median difference 5.0 mm and 3.8 mm). Diastolic and ceMRA diameters did not differ significantly. CMR diameters taken in systole showed no statistical difference to systolic diameters taken by cine-angiography, while diastolic and ceMRA diameters were significantly smaller. PPVI was feasible to a maximal CMR diameter of 31 mm measured by SSFP whole-heart sequence in systole. Absolute diameters of native RVOT differ depending on the CMR sequence and timing of acquisition (systolic vs diastolic gating). Diameters taken during heart catheterization by cine-angiography best correlate to systolic CMR values. Data may help to select RVOTs suitable for PPVI.



Similar content being viewed by others
References
Baumgartner H, Bonhoeffer P, De Groot NM, de Haan F, Deanfield JE, Galie N, Gatzoulis MA, Gohlke-Baerwolf C, Kaemmerer H, Kilner P, Meijboom F, Mulder BJ, Oechslin E, Oliver JM, Serraf A, Szatmari A, Thaulow E, Vouhe PR, Walma E, Task Force on the Management of Grown-up Congenital Heart Disease of the European Society of C, Association for European Paediatric C, Guidelines ESCCfP (2010) ESC Guidelines for the management of grown-up congenital heart disease (new version 2010). Eur Heart J 31(23):2915–2957. https://doi.org/10.1093/eurheartj/ehq249
Qureshi AM, Prieto LR (2015) Percutaneous pulmonary valve placement. Tex Heart Inst J 42(3):195–201. https://doi.org/10.14503/THIJ-14-4276
Khambadkone S, Nordmeyer J, Bonhoeffer P (2007) Percutaneous implantation of the pulmonary and aortic valves: indications and limitations. J Cardiovasc Med (Hagerstown) 8(1):57–61. https://doi.org/10.2459/01.JCM.0000247437.05194.8e
Momenah TS, El Oakley R, Al Najashi K, Khoshhal S, Al Qethamy H, Bonhoeffer P (2009) Extended application of percutaneous pulmonary valve implantation. J Am Coll Cardiol 53(20):1859–1863. https://doi.org/10.1016/j.jacc.2008.08.061
Boudjemline Y, Brugada G, Van-Aerschot I, Patel M, Basquin A, Bonnet C, Legendre A, Bonnet D, Iserin L (2012) Outcomes and safety of transcatheter pulmonary valve replacement in patients with large patched right ventricular outflow tracts. Arch Cardiovasc Dis 105(8–9):404–413. https://doi.org/10.1016/j.acvd.2012.05.002
Malekzadeh-Milani S, Ladouceur M, Cohen S, Iserin L, Boudjemline Y (2014) Results of transcatheter pulmonary valvulation in native or patched right ventricular outflow tracts. Arch Cardiovasc Dis 107(11):592–598. https://doi.org/10.1016/j.acvd.2014.07.045
Meadows JJ, Moore PM, Berman DP, Cheatham JP, Cheatham SL, Porras D, Gillespie MJ, Rome JJ, Zahn EM, McElhinney DB (2014) Use and performance of the Melody transcatheter pulmonary valve in native and postsurgical, nonconduit right ventricular outflow tracts. Circ Cardiovasc Interv 7(3):374–380. https://doi.org/10.1161/CIRCINTERVENTIONS.114.001225
Bertels RA, Blom NA, Schalij MJ (2010) Edwards SAPIEN transcatheter heart valve in native pulmonary valve position. Heart 96(9):661. https://doi.org/10.1136/hrt.2009.189944
Guccione P, Milanesi O, Hijazi ZM, Pongiglione G (2012) Transcatheter pulmonary valve implantation in native pulmonary outflow tract using the Edwards SAPIEN transcatheter heart valve. Eur J Cardiothorac Surg 41(5):1192–1194. https://doi.org/10.1093/ejcts/ezr130
Cools B, Brown SC, Heying R, Jansen K, Boshoff DE, Budts W, Gewillig M (2015) Percutaneous pulmonary valve implantation for free pulmonary regurgitation following conduit-free surgery of the right ventricular outflow tract. Int J Cardiol 186:129–135. https://doi.org/10.1016/j.ijcard.2015.03.108
Rockefeller T, Shahanavaz S, Zajarias A, Balzer D (2016) Transcatheter implantation of SAPIEN 3 valve in native right ventricular outflow tract for severe pulmonary regurgitation following tetralogy of fallot repair. Catheter Cardiovasc Interv 88(1):E28–33. https://doi.org/10.1002/ccd.26480
Haas NA, Carere RG, Kretschmar O, Horlick E, Rodes-Cabau J, de Wolf D, Gewillig M, Mullen M, Lehner A, Deutsch C, Bramlage P, Ewert P (2018) Early outcomes of percutaneous pulmonary valve implantation using the Edwards SAPIEN XT transcatheter heart valve system. Int J Cardiol 250:86–91. https://doi.org/10.1016/j.ijcard.2017.10.015
Esmaeili A, Bollmann S, Khalil M, De Rosa R, Fichtlscherer S, Akintuerk H, Schranz D (2018) Percutaneous pulmonary valve implantation for reconstruction of a patch-repaired right ventricular outflow tract. J Interv Cardiol 31(1):106–111. https://doi.org/10.1111/joic.12443
Boshoff DE, Cools BL, Heying R, Troost E, Kefer J, Budts W, Gewillig M (2013) Off-label use of percutaneous pulmonary valved stents in the right ventricular outflow tract: time to rewrite the label? Catheter Cardiovasc Interv 81(6):987–995. https://doi.org/10.1002/ccd.24594
Georgiev S, Tanase D, Ewert P, Meierhofer C, Hager A, von Ohain JP, Eicken A (2018) Percutaneous pulmonary valve implantation in patients with dysfunction of a "native" right ventricular outflow tract—mid-term results. Int J Cardiol 258:31–35. https://doi.org/10.1016/j.ijcard.2017.11.091
Schievano S, Coats L, Migliavacca F, Norman W, Frigiola A, Deanfield J, Bonhoeffer P, Taylor AM (2007) Variations in right ventricular outflow tract morphology following repair of congenital heart disease: implications for percutaneous pulmonary valve implantation. J Cardiovasc Magn Reson 9(4):687–695. https://doi.org/10.1080/10976640601187596
Schievano S, Migliavacca F, Coats L, Khambadkone S, Carminati M, Wilson N, Deanfield JE, Bonhoeffer P, Taylor AM (2007) Percutaneous pulmonary valve implantation based on rapid prototyping of right ventricular outflow tract and pulmonary trunk from MR data. Radiology 242(2):490–497. https://doi.org/10.1148/radiol.2422051994
Fratz S, Chung T, Greil GF, Samyn MM, Taylor AM, Valsangiacomo Buechel ER, Yoo SJ, Powell AJ (2013) Guidelines and protocols for cardiovascular magnetic resonance in children and adults with congenital heart disease: SCMR expert consensus group on congenital heart disease. J Cardiovasc Magn Reson 15:51. https://doi.org/10.1186/1532-429X-15-51
Fratz S, Schuhbaeck A, Buchner C, Busch R, Meierhofer C, Martinoff S, Hess J, Stern H (2009) Comparison of accuracy of axial slices versus short-axis slices for measuring ventricular volumes by cardiac magnetic resonance in patients with corrected tetralogy of fallot. Am J Cardiol 103(12):1764–1769. https://doi.org/10.1016/j.amjcard.2009.02.030
Martirosian P, Greil GF, Fenchel M, Kramer U, Miller S, Greiser A, Claussen CD, Schick F, Sieverding L (2007) Optimization of blood-myocardial contrast in 3D true FISP cardiac imaging at 1.5 T. Magn Reson Med 57(1):213–219. https://doi.org/10.1002/mrm.21099
Kondo C, Takada K, Yokoyama U, Nakajima Y, Momma K, Sakai F (2001) Comparison of three-dimensional contrast-enhanced magnetic resonance angiography and axial radiographic angiography for diagnosing congenital stenoses in small pulmonary arteries. Am J Cardiol 87(4):420–424. https://doi.org/10.1016/s0002-9149(00)01394-1
Francois CJ, Tuite D, Deshpande V, Jerecic R, Weale P, Carr JC (2008) Unenhanced MR angiography of the thoracic aorta: initial clinical evaluation. AJR Am J Roentgenol 190(4):902–906. https://doi.org/10.2214/AJR.07.2997
Potthast S, Mitsumori L, Stanescu LA, Richardson ML, Branch K, Dubinsky TJ, Maki JH (2010) Measuring aortic diameter with different MR techniques: comparison of three-dimensional (3D) navigated steady-state free-precession (SSFP), 3D contrast-enhanced magnetic resonance angiography (CE-MRA), 2D T2 black blood, and 2D cine SSFP. J Magn Reson Imaging 31(1):177–184. https://doi.org/10.1002/jmri.22016
Ruile P, Blanke P, Krauss T, Dorfs S, Jung B, Jander N, Leipsic J, Langer M, Neumann FJ, Pache G (2016) Pre-procedural assessment of aortic annulus dimensions for transcatheter aortic valve replacement: comparison of a non-contrast 3D MRA protocol with contrast-enhanced cardiac dual-source CT angiography. Eur Heart J Cardiovasc Imaging 17(4):458–466. https://doi.org/10.1093/ehjci/jev188
Gulani V, Calamante F, Shellock FG, Kanal E, Reeder SB, International Society for Magnetic Resonance in M (2017) Gadolinium deposition in the brain: summary of evidence and recommendations. Lancet Neurol 16(7):564–570. https://doi.org/10.1016/S1474-4422(17)30158-8
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
None of the authors have any conflict of interest to declare.
Ethical Approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Since study design was retrospective, no informed consent was needed.
Additional information
Publisher's Note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Ferrari, I., Shehu, N., Mkrtchyan, N. et al. Different CMR Imaging Modalities for Native and Patch-Repaired Right Ventricular Outflow Tract Sizing: Impact on Percutaneous Pulmonary Valve Replacement Planning. Pediatr Cardiol 41, 382–388 (2020). https://doi.org/10.1007/s00246-019-02270-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-019-02270-5