Abstract
Percutaneous treatment of patent ductus arteriosus (PDA) in extreme premature infants is technically difficult, and therefore, often not consider as an alternative to surgery. The main objective of our work was to compare respiratory status prior and post ductal closure and morbi-mortality, in our series of preterm infants with percutaneous PDA closure versus surgical ligation in the same time-period. Retrospective review of all premature infants submitted to percutaneous and surgical PDA closure from January 2011 to December 2016. All the antenatal, perinatal, and postnatal characteristics were collected. The main outcome was the assessment of the pulmonary status before and after ductal closure using a pulmonary score. Secondary outcomes included moderate-severe disability in neurodevelopment, death before discharge, moderate-severe chronic lung disease, and morbidity at discharge. 25 patients with a mean weight of 1330 g (± 280) underwent percutaneous closure of PDA with ADO-II-AS, and a total of 53 underwent surgical ligation. 28/53 with similar gestational age, birth weight, and procedure weight to those in the percutaneous group, were selected to perform the comparative study. Ductal closure (percutaneous and surgical) resulted in improved respiratory status. However, percutaneous group achieved a fastest respiratory improvement, than surgical group. The surgical closure group associated higher morbidity among survivors (HIV, number of sepsis, need, and duration of inotropics post-interventionism). The incidence of recurrent laryngeal nerve palsy among the surgical group was 17%. Percutaneous closure of PDA in carefully selected low-weight preterm infants is a safe and reliable alternative to surgical ligation.
Similar content being viewed by others
Introduction
Optimal treatment of patent ductus arteriosus (PDA) in preterm infants still carries huge controversy and debate. Traditionally, when the PDA does not close either spontaneously or in response to pharmacological treatment, the physician has 2 options: surgical closure of the ductus or to conservatively manage the possible adverse hemodynamic effects. The general consensus being that a PDA in a preterm infant should be closed to control problems associated with the persistence of the shunt such as: heart failure, prolonged dependency on mechanical ventilation [1, 2], hypotension (beyond the first 48 h of life) [3], pulmonary hemorrhage [4, 5] periventricular hemorrhage [6], necrotizing enterocolitis (NEC) [7], anomalies in cerebral perfusion [8], or to avoid future risks [9]. Despite this, disagreement still exists on timing and strategies for the management of PDA in preterm infants [10].
The objective of ductal ligation is the resolution of adverse hemodynamic effects of the left to right shunt. However, although successful ligation of the conduit leads to cessation of pulmonary overcirculation and systemic hypoperfusion, immediate and long-term adverse effects secondary to ductal ligation do exist, such as: hemodynamic instability secondary to left cardiac output diminishment and/or deterioration of global myocardial function [11,12,13], known as post-ligature syndrome (PLS); alteration in cerebral perfusion [14]; immediate diminishment in pulmonary compliancy post ductal ligation [15]; evidence of independent association between ligation and deterioration in neurodevelopment [16], higher incidence of bronchopulmonary dysplasia (BPD) [17]; vocal chord paralysis with an incidence varying from 5.2 to 67% [18,19,20,21,22]; association with chylothorax; and development of scoliosis, amongst others.
Recently, these complications have led to the consideration of other alternative strategies for ductal closure, such as closure by percutaneous interventionism. PDA closure, based on percutaneous approach, is one of the safest cardiac interventionist procedures and is considered procedure of choice in preterm infants ≥ 5 kg [23,24,25].
Percutaneous duct closure in preterm infants may offer an improved respiratory recovery, with less need for post-procedure respiratory support than surgical ligation [26, 27]. Several centers, including our institution, have recently reported PDA percutaneous occlusion in extremely premature infants [24, 28,29,30,31,32,33,34,35]. Different authors have documented excellent safety data with new generation devices like the Amplatzer Duct Occluder II Additional Size (ADO-II-AS), the Medtronic Microvascular Plug or the Amplatzer Vascular Plug-II (Table 1).
The main objective of this work was to compare respiratory status prior and post ductal closure, in our series of preterm infants with percutaneous closure and in our series of preterm infants with surgical closure over the same time-period and to evaluate the association between types of ductal closure (Percutaneous vs. surgical) using a pulmonary score.
The secondary objective was to compare the risk of BPD or death, or survival without associated morbidity (degree of intraventricular hemorrhage (IVH) ≥ 3, periventricular cystic leukomalacia, necrotizing enterocolitis (NEC) requiring surgery or peritoneal drainage, or retinopathy of prematurity (ROP) ≥ 3).
Materials and Methods
Population
A retrospective review was carried out of all preterm infants undergoing surgical ligation or percutaneous closure with ADO-II-AS of PDA, hospitalized in NICU in our center between Jan 2011 and Dec 2016. Our experience in percutaneous PDA closure in low-weight preterm infants has been previously described in the literature [34] (Fig. 1, example of percutaneous closure).
The percutaneous group included patients between 1 and 2 kg requiring continuous positive pressure respiratory support (invasive or non-invasive) at time of procedure. Patients with significant coexistent congenital heart disease and patients not requiring positive pressure ventilation were excluded.
The surgical group included the total of patients undergoing surgical ligation (via left lateral thoracotomy, between 3° and 4° intercostal space, with vascular clip) requiring positive pressure ventilation, including only patients with similar gestational age (GA), birth weight (BW), and procedure weight (WT) to those in the percutaneous group, to compare respiratory status prior and post ductal closure, and to compare the risk of BPD or death, or survival without associated morbidity.
Informed consent was obtained from all individual participants included in the study.
Pulmonary Score
Pulmonary scoring was used to evaluate basal pulmonary state in both study groups prior and post ductal closure. This was developed and validated in preterm infants included in the STOP-ROP trial (649 preterm infants) and was limited to descriptors of respiratory support retrospectively collected during the study. These descriptors included the use of medication during the week prior to randomization (methylxanthines, systemic or inhaled steroids, diuretics), quantity of supplementary oxygen, and type of ventilatory support. Relative weight (numerical value) for each item was decided by neonatologist clinical consensus. Greater importance was given to therapeutic interventions and these reflected greater degree of respiratory disease [36].
Pulmonary score was calculated as the Formula shown in Table 2. Pulmonary score may have a range between 0.21 (absence of pulmonary support, absence of oxygen, and no medication) and 2.95 (mechanical ventilation, Fi02 of 1, and receiving treatment with systemic steroids, diuretics, and methylxanthines).
To determine the basal state of the patient before PDA closure, pulmonary scores were calculated weekly, 4 weeks prior to procedure. Furthermore, to highlight the potential short and long-term benefits of PDA closure, pulmonary score was calculated weekly, 4 weeks post procedure (or until discharge), as well as immediately (2 days) post procedure.
Morbi-Mortality
Outcomes
Outcomes were: (i) BPD diagnosis (any oxygen therapy at 36 weeks of post-menstrual age), (ii) death before 36 weeks of PMA, and (iii) survival without significant neonatal morbidity (degree of intraventricular hemorrhage (HIV) ≥ 3, periventricular cystic leukomalacia, necrotizing enterocolitis (NEC) requiring surgery or peritoneal drainage, or retinopathy of prematurity (ROP) ≥ 3). Data for each preterm infant were collected until death or hospital discharge, (iv) Moderate-severe neurological impairment (NI).
Moderate to severe NI was defined as a compound of neuromotor, neurocognitive and/or neurosensorial deterioration. Surviving preterm infants underwent neurological development evaluation; clinical examination, visual and auditive evaluation, and cognitive evaluation using the Bayley Scale of Infant Development. Clinical examinations and the standard motor evaluations identified the presence of cerebral paralysis classified according to the Motor Function Classification System [38]. Cognition and language abilities were assessed mainly using BSID III at 12, 18 and 24 months of age.
Covariables
Covariables chosen for the analysis were: mother’s age, multiple pregnancy, preeclampsia/eclampsia, spontaneous onset of labor, premature membrane rupture, maternal infection as indication for delivery, prenatal steroids, cesarean section, gender; GA at birth, small for GA (classified as weight at birth < 3 SD or between percentiles 3 and 10 for GA and gender using Hadlock Reference) [39, 40], mechanical ventilation 28 days post procedure (surgical ligation or percutaneous closure), and number of septicemias confirmed by blood culture (≤ 1 o > 1).
Procedural and Follow-Up Complications of the Percutaneous Interventional Group
All complications related to the intervention procedure were recorded. A retrospective collection of clinical and echocardiographic data was performed for all patients, during hospital admission after percutaneous PDA closure, and after hospital discharge.
Other Complications
Incidence of vocal cord paralysis was also collected, and studied by direct laryngoscopy in the preterm infants with feeding difficulties, weaning from respiratory support difficulties, hoarseness, and developmental language problems.
Statistical Analysis
Standard descriptive statistics were used to summarize the data and expressed as median (range) for continuous variables and count (percentage of total) for categorical variables. Differences between groups were tested using t test for means comparison or Kruskal–Wallis test for continuous data, and χ 2 test for proportions. Statistical significance was established a priori using a two-tailed P value < 0.05. Associations between covariables and comorbidities, and type of ductal treatment were analysed using regression models, reported as Odds Ratio with confidence intervals of 95%.
A mixed lineal model with random interception was used to evaluate the tendency over time (effect of time) on mean pulmonary score of the cohort before and after ductal closure. This approach accounts for the correlation of measurements between different points on the same subject.
Results
Patient Characteristics
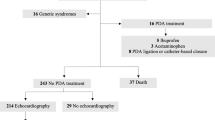
A total of 27 preterm infants undergoing percutaneous closure at our center were identified, two patients were excluded from the study due to relevant associated congenital heart disease. Patient and procedural characteristics were represented in Table 3. In the same time-period, a total of 53 preterm infants underwent PDA surgical ligation, 28 of these with weights between 1 and 2 kg at the time of procedure.
Prenatal and perinatal characteristics of infants treated percutaneously and surgically are shown in Table 4 (Total and Group between 1 and 2 kg).
Preterm infants undergoing surgical ligation had lower BWs than the preterm infants undergoing percutaneous closure (P = 0.033), higher maternal age (P = 0.012), and less ceserean section deliveries (P = 0.017), no differences were found amongst the other antenatal and perinatal items in both groups. However, when comparing the surgical group of 1 to 2 kg with the percutaneous group, the difference in weight at birth was no longer significant, only higher maternal age remained significant. Therefore, the percutaneous closure group and the surgical 1–2 kg group were homogeneous as regards GA at birth and weight at birth.
All patients, surgical and percutaneous, received a cycle of ibuprofen prior to closure indication. 92% of preterm infants in the percutaneous closure group received 2 cycles of treatment, as opposed to 79% in the surgical group (75% in the 1–2 kg group). Table 5 shows weight and GA on intervention in both treatment groups.
The preterm infants in the surgical ligation group had lower weight, GA, and age (days) on intervention than those in the percutaneous group (P < 0.001). However, when compared to the surgical 1–2 kg group on intervention, the weight difference on intervention was no longer significant, whereas lower GA on intervention and lower age in the surgical group remained significant, with a difference of 1.2 weeks and 8 days of life, 95% CI of 0.003 to 2.32 and 0.9 to 15.5.
Comparative Study: Percutaneous Closure Versus Surgical Closure (Group 1–2 kg)
Pulmonary Score
Figure 2a, b, show the trend of pulmonary status change prior to and post intervention in the Percutaneous group and Surgical group. In the percutaneous group (Fig. 2a), although no significant change was observed prior to catheterization, pulmonary score varied with time before and after ductal closure (Fig. 2, P < 0.001, global), increasing until the moment of closure and decreasing after closure. Furthermore, the time variable (passing of time) only explained this variation of pulmonary score in 28%, revealing a significative variation, between the pre-procedure measurement (day − 2), from day + 7 post procedure.
Pulmonary score pre- and post-cardiac catheterization (a) and pre- and post-surgical ligation (b). X-axis represents time (days), with negative values denoting days prior to catheterization. Y-axis designates Pulmonary Scores (mean). Table designates Pulmonary score (Maximum, p75, mean, p25, minimum). Pulmonary scores decreased following PDA closure (linear mixed-effect model, P < 0.001) in both groups
The variation with time of pulmonary score in the surgical group was significant (Fig. 2b, P < 0.001, global). Furthermore, the time variable (passing of time) only explained this variation in pulmonary score in 19%. Likewise, this decrease in pulmonary score does not occur until + 7 post ductal closure (the graph shows the increase in pulmonary score on day + 2 post procedure compared to previous status, showing a significant improvement, between pre-procedure measurements (day − 2), and from day + 14 post procedure (P = 0.045).
When comparing the different measurements of pulmonary score in the different time intervals between the surgical and percutaneous groups, taking the percutaneous group as reference, no significant differences were observed in scores prior to ductal closure, with a trend towards increase in pulmonary score until PDA closure in both groups. However, in the post ductal closure, there is an earlier improvement in pulmonary status in the percutaneous group, which begins immediately post ductal closure. This difference is significant above all on days + 7 and + 14 post closure, whether compared to the surgical group (Fig. 3).
Comparison of pulmonary score before and after PDA closure (red: surgical ligation; blue: percutaneous closure). X-axis represents time (days), with negative values denoting days prior to surgery. Y-axis designates pulmonary scores (mean). Table designates the mean differences (95% CI, P value). Reference group: percutaneous closure
Morbi-Mortality
Preterm infants undergoing surgical ligation had higher morbidity rates during the postnatal period than the percutaneous closure group (Table 6). This is statistically significant for IVH 3–4 (P = 0.024) and inotropic therapy duration post intervention (P < 0.001).
Outcomes
In the univariate model (model 1), percutaneous PDA closure was associated to lower hospital mortality rates before 36 weeks of PMA (Difference of − 17% in the percutaneous group with P = 0.045, OR 0.15.) No significant differences were found for the remaining variables (BPD, Morbidity at discharge and moderate-severe neurodevelopmental impairment). After adjusting the model for the antenatal, perinatal, and postnatal variables (covariables) in Model 2, no relationship was found between percutaneous closure and lower hospital mortality before 36 weeks (Table 7).
Procedural and Follow-Up Complications of the Percutaneous Interventional Group
There were no major complications during the procedure. Three patients had a small residual shunt (< 3 mm), observed by angiography and echocardiography. In 4 patients (15%), a slight protrusion of the device was observed in the left pulmonary artery (LPA) after its release. Two patients suffer device embolization. In both patients, the device migrated to the left pulmonary artery, and was recovered without complications in the same procedure, achieving successful closure, oversizing the device waist diameter by 1 mm. Twelve patients (48%) required blood transfusion immediately post procedure. Renal function was preserved in all cases, despite using contrast angiography (median contrast volume 7 ml/kg). One patient died secondary to sepsis, not secondary to PDA pathology, or related to the procedure.
Mean follow-up time of those who survived to hospital discharge was 38 months (8–73 months). Complete PDA occlusion without aortic arch or LPA stenosis, with normal pulmonary pressures, was observed in all patients on follow-up. The 4 patients who presented LPA protrusion, producing slight stenosis solved in the following controls, demonstrating adequate growth of the vessel within 3–6 months. Despite being a cohort of very low-weight patients, no complications related to vascular access were detected. In our experience, optimal control of the aortic arch was obtained in recirculation, without the need to obtain arterial access, limiting vascular damage.
Other Complications
The principle cause of mortality in the surgical group was sepsis (95%), as well as higher rates of early respiratory failure. Rates for recurrent vocal cord paralysis in the surgical group were 17% and no cases in the percutaneous group.
No significant association was found between PDA size (recorded by echo) and pulmonary score (pre- and post-PDA closure), moderate-severe disability in neurodevelopment, death before discharge, moderate-severe chronic lung disease, and morbidity at discharge.
Discussion
Percutaneous treatment of PDA in preterm infants under 2 kg is technically complex due to the size of delivery sheaths for occlusion devices, the size of vascular structures adjacent to PDA, as well as the size and morphology of the PDA itself (moderate size tubular PDA). Complications described include: residual short-circuits, embolization, and obstruction of aorta and left pulmonary artery. Therefore, percutaneous PDA closure is not a routine alternative for surgery when medical treatment fails. Nevertheless, the secondary effects thoracotomy may cause in these patients should not be underestimated, lung edema, bronchial obstruction, diaphragmatic paresia, thorax deformation, recurrent vocal cord paralysis, chylothorax, and/or long-term sequelae like scoliosis [41, 42]. These considerations have led to a tendency to avoid definitive treatment and to a permissive approach to PDA in preterm infants; fluid restriction, diuretics, and positive pressure increase at end of expiration [12, 43]. Although various studies have reported no worse outcomes, and in some cases better results than with surgical ligation [44, 45], more recent studies have associated this approach with an increase in morbidity and mortality [46, 47]. The lack of an ideal evidence-based therapy for treatment of PDA in preterm infants led our group to explore the possibility of performing percutaneous closure of PDA in this population.
Pulmonary Status
There has been a recent shift in the management of PDA in preterm infants. Meta-analysis of clinical trial literature on treatment of ductus shows that early treatment may not be beneficial, and conservative treatment may encourage better mid to long-term results, limiting ductus treatment to those whose clinical, hemodynamic, and/or respiratory evolution is not favorable. As seen in our cohort of preterm infants receiving surgical or percutaneous treatment, evolution of respiratory status until the moment of intervention, far from improving, suffers progressive worsening until the moment of closure, and after closure, begins to improve. To tackle the fundamental questions regarding clinical and/or hemodynamic thresholds for PDA surgical or percutaneous closure, large, multi-centric studies are necessary to detect clinically significant differences in results in a range of weighted categories. Furthermore, consideration of specific factors beyond weight (preterm, congenital anomalies, comorbidity) underlying the risk are fundamental for the development of risk stratification models.
These data support the idea that percutaneous PDA closure in preterm infants with significant respiratory support is effective with a safety profile comparable to surgical ligation. Furthermore, patients undergoing percutaneous closure, in our series, recovered respiratory status earlier than the surgical group. A decrease in pulmonary score was appreciated immediately post ductal closure and improved significantly from the first week post procedure, as opposed to the surgical group, in which an initial rise in the post-procedure score was observed, and no significant improvement was observed until the second week post procedure, which could indicate and confirm the effects of thoracotomy and possible lung edema produced by the surgical ligation technique. These data, however, should be interpreted with caution, as the higher rates of mortality and morbidity in the surgical group, even long after successful PDA closure, reflect the complicated baseline medical situation and the multiple comorbidities leading to prolonged positive pressure ventilation after birth.
Morbi-Mortality
In this study, we also tried to establish whether the percutaneous approach would be less likely than the surgical approach to produce signs and symptoms of cardiac compromise, commonly known as post ligation syndrome (PLS), as well as the development of BPD (requiring 02 at 36 weeks of PMA), development of NEC requiring surgical revision or grade > 2, ROP ≥ grade 3, IVH grade 3–4 and/or cPVL (comorbidity at discharge amongst survivors), lower probabilities of moderate-severe neurodevelopmental impairment on evolution, and lower probabilities for hospital death before week 36 of PMA. As well as the incidence of problems commonly associated with surgical ligation like recurring vocal cord paralysis.
In this study, the percutaneous group required less use of inotropic therapy post procedure and for shorter intervals when used. Patients with PLS suffer hemodynamic instability, an increase in need for inotropic therapy and an increase in respiratory support in the immediate 24 h post ductus ligation. Etiology of PLS is not well understood, but probably includes the incapacity of the premature neonatal myocardium to tolerate sudden increases in post load resulting in deterioration of left ventricle performance and lung edema. The mechanical manipulation of the left lung, necessary in surgical ligation, may also contribute to this clinical scenario.
In this study of a retrospective cohort of preterm infants with hemodynamically significant PDA, in whom an intervention for closure was performed, lower probabilities for development of comorbidities were observed, when compared to the surgical group with lower rates for the percutaneous group which could indicate a protective effect for percutaneous approach as opposed to the surgical. However, in the multi-variate analysis, including prenatal, perinatal, and postnatal variables, this protective association could not be proved, so we demonstrate association, rather than causality. As previously mentioned, to tackle these fundamental questions regarding which strategy to use in PDA closure, large, multi-centric studies with sufficient power are needed to detect differences clinically, furthermore, the consideration of specific patient factors underlying risk, is essential for the development of risk stratification models.
Feasibility of the Percutaneous Approach
Percutaneous PDA treatment in preterm infants with weights below 2 kg is technically complex, due to the size of the delivery device systems; the size of the vascular structures adjacent to the PDA, and the PDA size and morphology found in most of these patients, with complications described such as residual shunts, embolizations, vascular damage, and obstruction of the aorta and left pulmonary artery. Therefore, percutaneous PDA closure is not routinely considered as an alternative to surgery when medical treatment fails. However, percutaneous PDA closure in this context, with the emergence of new devices and improvement in the learning curve, supported by recent works (Table 1) [24, 28,29,30,31,32,33,34,35], has shown near 100% occlusion success rate, with a low rate of complications, demonstrating similar incidences of adverse events as their homologous with higher weights [48]. Therefore, percutaneous PDA closure approach in preterm infants with very low BW, appears to be a promising option, to reduce the consequences of conservative therapy and to avoid the possible adverse effects of left thoracotomy.
Limitations
This study is retrospective and the assignation of treatment strategy was not randomized. Although, we attempted to control factors which could bias results, other differences between the study groups may exist and therefore, a definitive causal relationship could not be established between the method of PDA closure and respiratory function recovery time as well as and morbi-mortality probability.
Even in an institution with a focus on preterm PDA management, the decision to refer for percutaneous closure or not, lies with the attending physician, with marked variation in referral timing for percutaneous closure. Thus, questions relative to selection and optimal timing for percutaneous closure remain unanswered. Although currently there is no standard criteria to determine which preterm infants should undergo PDA closure, all the study patients treated with percutaneous closure fulfilled the surgical criteria.
Given that this study was carried out in a highly specialized center, the risk of reference bias is acknowledged.
Although vascular lesions were not observed during follow-up (clinical examination), vascular echo-doppler was not performed in all patients in the percutaneous group, so we could not safely state that there were no problems with vascular access.
As previous data have proven that mechanical ventilation increases risk of chronic lung disease in very premature infants, the improved respiratory state post percutaneous closure is encouraging and adds to the increasing proof on the potential value of this approach [23]. However, we recognize that the possibility of non-measured effects, including improvement of respiratory function over time (nutrition, lineal growth), may contribute to clinical improvement.
Conclusion
The positive impact of percutaneous PDA closure on pulmonary recovery post intervention is greater than with surgical closure with equal weights and GAs, showing an early recovery and less need for short-term respiratory support.
The negative impact of PDA closure in preterm infants on hemodynamics is lower in the percutaneous group with equal weights and GAs, proving less need for inotropic support post ligation.
Percutaneous ductal closure associates lower morbidity and lower mortality when compared to the surgical group. However, more controlled clinical trials are necessary to evaluate the clinically relevant differences in results post percutaneous approach of ductal closure versus alternative management strategies (surgical ligation).
References
Marshall DD, Kotelchuck M, Young TE et al (1999) Risk factors for chronic lung disease in the surfactant era: a North Carolina population-based study of very low birth weight infants. North Carolina Neonatologists Association. Pediatrics 104:1345–1350
Brown ER (1979) Increased risk of bronchopulmonary dysplasia in infants with patent ductus arteriosus. J Pediatr 95:865–866
Sarkar S, Dechert R, Schumacher RE (2007) Is refractory hypotension in preterm infants a manifestation of early ductal shunting? J Perinatol 27:353–358
Garland J, Buck R, Weinberg M (1994) Pulmonary hemorrhage risk in infants with a clinically diagnosed patent ductus arteriosus: a retrospective cohort study. Pediatrics 94:719–723
Kluckow M, Evans N (2000) Ductal shunting, high pulmonary blood flow, and pulmonary hemorrhage. J Pediatr 137:68–72
Evans N, Kluckow M (1996) Early ductal shunting and intraventricular haemorrhage in ventilated preterm infants. Arch Dis Child Fetal Neonatal Ed 75:F183–F186
Dollberg S, Lusky A, Reichman B (2005) Patent ductus arteriosus, indomethacin and necrotizing enterocolitis in very low birth weight infants: a population-based study. J Pediatr Gastroenterol Nutr 40:184–188
Lemmers PM, Toet MC, van Bel F (2008) Impact of patent ductus arteriosus and subsequent therapy with indomethacin on cerebral oxygenation in preterm infants. Pediatrics 121:142–147
Heuchan AM, Clyman RI (2014) Managing the patent ductus arteriosus: current treatment options. Arch Dis Child Fetal Neonatal Ed 99(5):F431–F436
Hamrick SE, Hansmann G (2010) Patent ductus arteriosus of the preterm infant. Pediatrics 125:1020–1030
Noori S, Friedlich P, Seri I et al (2007) Changes in myocardial function and hemodynamics after ligation of the ductus arteriosus in preterm infants. J Pediatr 150:597–602
Noori S (2010) Patent ductus arteriosus in the preterm infant: to treat or not to treat? J Perinatol 30:S31–S37
McNamara PJ, Stewart L, Shivananda SP et al (2010) Patent ductus arteriosus ligation is associated with impaired left ventricular systolic perfor mance in premature infants weighing less than 1000 g. J Thorac Cardiovasc Surg 140:150–157
Zaramella P, Freato F, Quaresima V et al (2006) Surgical closure of patent ductus arteriosus reduces the cerebral tissue oxygenation index in pre- term infants: a near-infrared spectroscopy and Doppler study. Pediatr Int 48:305–312
Teixeira LS, Shivananda SP, Stephens D et al (2008) Postoperative cardiore-spiratory instability following ligation of the preterm ductus arteriosus is related to early need for intervention. J Perinatol 28:803–810
Madan JC, Kendrick D, Hagadorn JI et al (2009) Patent ductus arteriosus therapy: impact on neonatal and 18-month outcome. Pediatrics 123:674–681
Clyman R, Cassady G, Kirklin JK et al (2009) The role of patent ductus arteriosus ligation in bronchopulmonary dysplasia: reexamining a ran- domized controlled trial. J Pediatr 154:873–876
Davis JT, Baciewicz FA, Suriyapa S et al (1988) Vocal cord paralysis in pre- mature infants undergoing ductal closure. Ann Thorac Surg 46:214–215
Zbar RI, Chen AH, Behrendt DM et al (1996) Incidence of vocal fold paralysis in infants undergoing ligation of patent ductus arteriosus. Ann Thorac Surg 61:814–816
Pereira KD, Webb BD, Blakely ML et al (2006) Sequelae of recurrent laryngeal nerve injury after patent ductus arteriosus ligation. Int J Pediatr Otorhinolaryngol 70:1609–1612
Clement WA, El-Hakim H, Phillipos EZ et al (2008) Unilateral vocal cord paralysis following patent ductus arteriosus ligation in extremely low- birth-weight infants. Arch Otolaryngol Head Neck Surg 134:28–33
Benjamin JR, Smith PB, Cotten CM et al (2010) Long-term morbidities associated with vocal cord paralysis after surgical closure of a patent ductus arteriosus in extremely low birth weight infants. J Perinatol 30:408–413
Backes CH, Cheatham SL, Deyo GM et al (2016) Percutaneous patent ductus arteriosus (PDA) closure in very preterm infants: feasibility and complications. J Am Heart Assoc 5(2):e002923
Sathanandam S, Justino H, Waller BR et al (2017) Initial clinical experience with the Medtronic Micro Vascular Plug™ in transcatheter occlusion of PDAs in extremely premature infants. Catheter Cardiovasc Interv 89(6):1051–1058
Dimas VV, Takao C, Ing FF et al (2010) Outcomes of transcatheter occlusion of patent ductus arteriosus in infants weighing ≤ 6 kg. JACC Cardiovasc Interv 3:1295–1299
Chen ZY, Wu LM, Luo YK et al (2009) Comparison of long-term clinical outcome between trans- catheter Amplatzer occlusion and surgical closure of isolated patent ductus arteriosus. Chin Med J 122:1123–1127
Abu Hazeem AA, Gillespie MJ, Thun H et al (2013) Percutaneous closure of patent ductus arteriosus in small infants with significant lung disease may offer faster recovery of respiratory function when compared to surgical ligation. Catheter Cardiovasc Interv 82(4):526–533
Francis E, Singhi AK, Lakshmivenkateshaiah S et al (2010) Transcatheter occlusion of patent ductus arteriosus in pre-term infants. JACC Cardiovasc Interv 3:550–555
Roberts P, Adwani S, Archer N et al (2007) Catheter closure of the arterial duct in preterm infants. Arch Dis Child Fetal Neonatal Ed 92:F248–F250
Zahn EM, Nevin P, Simmons C et al (2015) A novel technique for transcatheter patent ductus arteriosus closure in extremely pre- term infants using commercially available technology. Catheter Cardiovasc Interv 85:240–248
Bentham J, Meur S, Hudsmith L et al (2011) Echocardiographically guided catheter closure of arterial ducts in small preterm infants on the neonatal intensive care unit. Catheter Cardiovasc Interv 77:409–415
Philip R, Rush Waller B, Agrawal V et al (2016) Morphologic characterization of the patent ductus arteriosus in the premature infant and the choice of transcatheter occlusion device. Catheter Cardiovasc Interv 87:310
Narin N, Pamukcu O, Baykan A et al (2016) Percutaneous PDA closure in extremely low birth weight babies. J Interv Cardiol 29(6):654–660
Rodríguez A, Ballesteros F, Blanco D et al (2017) Trascatheter occlusion of patent ductus arteriosus in preterm infants weighing less than 2 kg with the amplatzer duct occluder II additional sizes device. Rev Esp Cardiol. https://doi.org/10.1016/j.rec.2017.08.014
Morville P, Akhavi A (2017) Transcatheter closure of hemodynamic significant patent ductus arteriosus in 32 premature infants by amplatzer ductal occluder additional size-ADOIIAS. Catheter Cardiovasc Interv. https://doi.org/10.1002/ccd.27091
Benaron DA, Benitz WE (1994) Maximizing the stability of oxygen delivered via nasal cannula. Arch Pediatr Adolesc Med 148:294–300
Madan A, Brozanski BS, Cole CH et al (2005) A pulmonary score for assessing the severity of neonatal chronic lung disease. Pediatrics 115:e450–e457
Palisano R, Rosenbaum P, Walter S et al (1997) Development and reliability of a system to classify gross motor function in children with cerebral palsy. Dev Med Child Neurol 39(4):214–223
Mikolajczyk RT, Zhang J, Betran AP et al (2011) A global reference for fetal-weight and birthweight percentiles. Lancet 377:1855–1861
de Jong CL, Gardosi J, Baldwin C et al (1998) Fetal weight gain in a serially scanned high-risk population. Ultrasound Obstet Gynecol 11:39–43
Noori S (2012) Pros and cons of patent ductus arteriosus ligation: hemodynamic changes and other morbidities after patent ductus arteriosus ligation. Sem Perinatol 36(2):139–145
Trotter A, Larsch FJ, Hannekum A et al (2009) Thoracic alterations after surgical closure of the ductus arteriosus botallo in preterm infants. Klin Padiatr 221(4):227–231
Benitz WE (2012) Patent ductus arteriosus: to treat or not to treat? Arch Dis Child Fetal Neonatal Ed 97:F80–F82
Wickremasinghe AC, Rogers EE, Piecuch RE et al (2012) Neurodevelopmental outcomes following two different treatment approaches (early ligation and selective ligation) for patent ductus arterio- sus. J Pediatr 161:1065–1072
Vanhaesebrouck S, Zonnenberg I, Vandervoort P et al (2007) Conservative treatment for patent ductus arteriosus in the preterm. Arch Dis Child Fetal Neonatal Ed 92:F244–F247
Kaempf JW, Wu YX, Kaempf AJ et al (2012) What happens when the patent ductus arte- riosus is treated less aggressively in very low birth weight infants? J Perinatol 32:344–348
Noori S, McCoy M, Friedlich P et al (2009) Failure of ductus arteriosus closure is associated with increased mortality in preterm infants. Pediatrics 123:e138–e144
Backes CH, Kennedy KF, Locke M et al (2017) Transcatheter occlusion of the patent ductus arteriosus in 747 infants < 6 kg: insights From the NCDR IMPACT registry. JACC Cardiovasc Interv 10(17):1729–1737
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare they have no conflict of interest.
Ethical Approval
All procedures performed in this study involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed Consent
Informed consent was obtained from all individual participants included in the study.
Research Involving Human Participants and/or Animals
This article does not contain any studies with animals performed by any of the authors.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Rodríguez Ogando, A., Planelles Asensio, I., de la Blanca, A.R.S. et al. Surgical Ligation Versus Percutaneous Closure of Patent Ductus Arteriosus in Very Low-Weight Preterm Infants: Which are the Real Benefits of the Percutaneous Approach?. Pediatr Cardiol 39, 398–410 (2018). https://doi.org/10.1007/s00246-017-1768-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-017-1768-5