Abstract
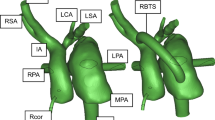
The central aortic shunt, consisting of a Gore-Tex (polytetrafluoroethylene) tube (graft) connecting the ascending aorta to the pulmonary artery, is a palliative operation for neonates with cyanotic congenital heart disease. These tubes often have an extended length, and therefore must be angulated to complete the connection to the posterior pulmonary arteries. Thrombosis of the graft is not uncommon and can be life-threatening. We have shown that a viscous fluid (such as blood) traversing a curve or bend in a small-caliber vessel or conduit can give rise to marked increases in wall shear stress, which is the major mechanical factor responsible for vascular thrombosis. Thus, the objective of this study was to use computational fluid dynamics to investigate whether wall shear stress (and shear rate) generated in angulated central aorta-to-pulmonary artery connections, in vivo, can be of magnitude and distribution to initiate platelet activation/aggregation, ultimately leading to thrombus formation. Anatomical features required to construct the computer-simulated blood flow pathways were verified from angiograms of central aortic shunts in patients. For the modeled central aortic shunts, we found wall shear stresses of (80–200 N/m2), with shear rates of (16,000–40,000/s), at sites of even modest curvature, to be high enough to cause platelet-mediated shunt thrombosis. The corresponding energy losses for the fluid transitions through the aorta-to-pulmonary connections constituted (70 %) of the incoming flow’s mechanical energy. The associated velocity fields within these shunts exhibited vortices, eddies, and flow stagnation/recirculation, which are thrombogenic in nature and conducive to energy dissipation. Angulation-induced, shear stress-mediated shunt thrombosis is insensitive to aspirin therapy alone. Thus, for patients with central aortic shunts of longer length and with angulation, aspirin alone will provide insufficient protection against clotting. These patients are at risk for shunt thrombosis and significant morbidity and mortality, unless their anticoagulation regimen includes additional antiplatelet medications.











Similar content being viewed by others
References
Al Jubair KA, Al Fagih MR, Al Jarallah AS, Al Yousef S, All Khan MA, Ashmeg A, Al Faraldi Y, Sawyer W (1998) Results of 546 Blalock-Taussig shunts performed in 478 patients. Cardiol Young. 8(4):486–490
Amato JJ, Marbey ML, Bush C, Galdieri RJ, Cotroneo JV, Bushong J (1988) Systemic-pulmonary polytetrafluoroethylene shunts in palliative operations for congenital heart surgery. Revival of the central shunt. J Thorac Cardiovasc Surg. 95:62–69
Ascuitto RJ, Kydon DW, Ross-Ascuitto NT (2001) Pressure loss from flow energy dissipation: relevance to Fontan-type modifications. Pediatr Cardiol 22:110–115
Ascuitto R, Ross-Ascuitto N, Guillot M (2010) Fluid Viscosity increases pressure drop and exacerbates flow-energy loss (Relevance to Modified-Fontan patients with elevated hematocrit). Congenit Cardiol Today 8(12):3–12
Barragry TP, Ring WS, Blatchford JW, Foker JE (1987) Central aorta-pulmonary artery shunts in neonates with complex cyanotic congenital heart disease. J Thorac Cardiovasc Surg. 93:767–774
Berger SA, Talbot L, Yao LS (1983) Flow in curved pipes. Ann Rev Fluid Mech 15:461–512
Bove EL, Sondheimer HM, Kavey REW, Byram CJ, Blackman MS, Parker FB Jr (1984) Subclavian-pulmonary artery shunts with polytetrafluoroethylene interposition grafts. Ann Thorac Surg 37:88–91
Ciaravella JM, Midgley FM (1980) Construction of interposition polytetrafluoroethylene ascending aorta-pulmonary artery shunt. Ann Thorac Surg 29:570–572
Cook NS, Kottirsch G, Zerwes H-G (1994) Platelet glycoprotein IIb/IIIa antagonists. Drugs Future. 19:135–159
Davidson JS (1955) Anastomosis between the ascending aorta and the main pulmonary artery in the tetralogy of Fallot. Thorax. 10:348–350
Dean WR (1927) Note on the motion of fluid in a curved pipe. Phil Mag J 20:208–223
Dean WR (1928) LXXII. The stream-line motion of fluid in a curved pipe (Second Paper). Phil Mag S 7 Ser 5(30):673–695
Donahoo JS, Gardner TJ, Zahka K, Kidd L (1980) Systemic-pulmonary shunts in neonates and infants using microporous expanded polytetrafluoroethylene: Immediate and late results. Ann Thorac Surg 30:146–150
Fruccone NJ, Bowman FO, Malm JR, Gersony WM (1974) Systemic-pulmonary arterial shunts in the first year of life. Circulation 49:508–511
Gazzaniga AB, Elliott MP, Sperling DR et al (1976) Microporous expanded polytetrafluoroethylene arterial prosthesis for construction of aortopulmonary shunts; experimental and clinical results. Ann Thorac Surg 21:322–327
Goto S, Salomon DR, Ikeda Y, Ruggeri ZM (1995) Characterization of the unique mechanisms mediating the shear-dependent binding of soluble von Willebrand factor to platelets. J Biol Chem 270:23353
Guan X, Martonen TB (1997) Simulations of flow in curved tubes. Aerosol Sci Technol 26:485–504
Guillot M, Ross-Ascuitto N, Ascuitto R (2012) Fluid-flow energetics for curved or angulated pathways associated with staged operations for the modified Fontan procedure. Congenit Cardiol Today 10(1):2–14
Guyton RA, Owens JE, Waumett JD, Dooley KJ, Hatcher CR, Williams WH (1983) The Blalock-Taussig shunt. J Thorac Cardiovasc Surg 85:917–922
Hathcock JJ (2006) Flow effects on coagulation and thrombosis. Arterioscler Thromb Vasc Biol 26:1729–1737
Heyligers JMM, Verhagen HJM, Rotmans JI, Weeterings C, deGroot PG, Moll FL, Lisman T (2006) Heparin immobilization reduces thrombogenicity of small-caliber expanded polytetrafluoroethylene grafts. J Vasc Surg 43(3):587–591
Holme PA, Orvim LL, Hamers MJAG, Solum NO, Brosstad FR, Barstad RM, Sakariassen KS (1997) Shear-induced platelet activation and platelet microparticle formation at blood flow conditions as in arteries with severe stenosis. Arterioscler Thromb Vasc Biol 17:646–653
Jennings RB, Innes BJ, Brickman RD (1978) Use of microporous expanded polytetrafluoroethylene grafts for aorta-pulmonary shunts in infants and complex cyanotic heart disease. J Thorac Cardiovasc Surg 76:489–494
Kroll MH, Hellums JD, McIntire LV, Schafer AI, Moake JL (1996) Platelets and shear stress. Blood. 88:1525–1541
Kulik TJ, Foker JE, Lucas RV, et al (1981) Post-operative hemodynamics in children with polytetrafluoroethylene shunts. Circulation 64(Pt 2):II123-130
Laks H, Fagan L, Barner HB, Willman VL (1978) The Blalock–Taussig shunt in the neonate. Ann Thorac Surg 25:220–224
Lamberti JJ, Carlisle J, Waldman JD et al (1984) Systemic-pulmonary shunts in infants and children. J Thorac Cardiovasc Surg 88:76–81
Li JS, Yow E, Berezny KY, Rhodes JF, Bokesch PM, Charpie JR et al (2007) Clinical outcomes of palliative surgery including a systemic-to-pulmonary artery shunt in infants with cyanotic congenital heart disease. Does aspirin make a difference? Circulation. 116:293–297
Maalej N, Folts JD (1996) Increased shear stress overcomes the antithrombotic platelet inhibitory effect of aspirin in stenosed dog coronary arteries. Circulation 93:1201–1205
Martin D, Zaman A, Hacker J, Mendelow D, Birchall D (2009) Analysis of haemodynamic factors involved in carotid atherosclerosis using computational fluid dynamics. Br J Radiol 82:S33–S38
McCrary JK, Nolasco LH, Hellums JD, Kroll MH, Turner NA, Moake JL (1995) Direct demonstration of radiolabeled von Willebrand factor binding to platelet glycoprotein Ib and IIb–IIIa in the presence of shear stress. Ann Biomed Eng 23:787
Migliavacca F, Dubini G, Pennati G, Pietrabissa R, Fumero R, Hsia T-Y, de Leval MR (2000) Computational model of the fluid dynamics in systemic-to-pulmonary shunts. J Biomech 33:549–557
Miyazaki Y, Nomura S, Miyake T, Kagawa H, Kitada C, Taniguchi H, Komiyama Y, Fujimura Y, Ikeda Y, Fukuhara S (1996) High shear stress can initate both platelet aggregation and shedding of procoagulant containing microparticles. Blood 88:3456–3464
Motz R, Wessel A, Ruschewski W, Bursch J (1999) Reduced frequency of occlusion of aorto-pulmonary shunts in infants receiving Aspirin. Cardiol Young. 9:474–477
Mousa SA, Bennett JS (1996) Platelets in health and disease: platelet GPIIb/IIIa structure and function: recent advances in antiplatelet therapy. Drugs Future. 21:1141–1154
Moyle KR, Mallinson GD, Occleshaw CJ, Cowan BR, Gentles TL (2006) Wall shear stress is the primary mechanism of energy loss in the Fontan connection. Pediatr Cardiol 27:309–315
Munson BR, Young DF, Okiishi TH (2002) Fundamentals of fluid mechanics, 4th edn. Wiley, New York, pp 350–352
Nakamura T, Uchiyama S, Yamazaki M, Iwata M (2002) Effects of dipyridamole and aspirin on shear-induced platelet aggregation in whole blood and platelet-rich plasma. Cerebrovasc Dis 14:234–238
Nygard KK, Wilder M, Berkson J (1935) The relation between viscosity of the blood and the relative volume of erythrocytes (hematocrit value). Am. J Physiol 114:128–131
O’Brien JR (1990) Shear-induced platelet aggregation (Review Article). The Lancet. 335:711–713
Ohuchi H, Okabe H, Nagata N, Koseni K, Kaneko Y, Itoh K (1996) Long-term patency after the Blalock-Taussig operation-comparison between classic and modified shunts. Nihon Kyobugeka Gakkai Zasshi (J Jpn Assoc Thorac Surg) 44:1108–1113
Redo SF, Ecker RR (1963) Intrapericardial aortico-pulmonary artery shunt. Circulation 28:520–524
Ruggeri ZM, Orje JN, Haberman R, Federici AB, Reininger AJ (2006) Activation-independent platelet adhesion and aggregation under elevated shear stress. Blood 108(6):1903–1910
Schror K (1993) The basic pharmacology of Ticlopidine and Clopidogrel. Platelets. 4(5):252–261
Shanmugavelayudam SK, Rubenstein DA, Yin W (2011) Effects of physiologically relevant dynamic shear stress on platelet complement activation. Platelets 22:602–610
Siggers JH, Waters SL (2005) Steady flows in pipes with finite curvature. Am Inst Phys Phys Fluids. 17(7):07102
Song M-H, Sato M, Ueda Y (2001) Three-dimensional simulation of the Blalock-Taussig shunt using computational fluid dynamics. Surg Today. 31:688–694
Takahashi M, Mason WH, Weiner K, McCurdy DK, Berdjis F, Peoples WM (2003) Changes in coronary artery aneurysms following treatment with Abciximab. Pediatr Res 53:164
Waniewski J, Kurowska W, Mizerski JK, Trykozko A, Nowinski K, Brzezinska-Rajszys G, Kosciesza A (2005) The effects of graft geometry on the patency of a systemic-to-pulmonary shunt: a computational fluid dynamics study. Artif Organs 29(8):642–650
Wessel DL, Berger F, Li JS, Dahnert I, Rakhit A, Fontecave S, Newberger JW, for the CLARINET Investigators (2013) Clopidogrel in infants with systemic-to-pulmonary-artery shunts. N Engl J Med 368:2377–2384
Woolf PK, Stephenson LW, Meijboom E et al (1984) A comparison of Blalock-Taussig, Waterston, and polytetrafluoroethylene shunts in children less than two weeks of age. Ann Thorac Surg 38:26–30
Acknowledgments
This research was supported by a grant from the Board of Regents Grant: LSEQF-RD-A-18 Support Fund, State of Louisiana.
Conflicts of interest
There are no conflicts of interest to disclose.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Curvature is a measure of how sharply a curve bends. Curvature is zero for a straight line, small in value for curves which bend little and large for curves which angle sharply. The equation for a symmetric parabola, restricted to the Cartesian (x,y) plane, and with vertex at the origin (0,0), has the simple form y = x 2/4f, where f (referred to as the focal length) is a constant describing the degree of curvature. The curvature (K) at a point (x) along the curve is given by K(x) = [4f 2/(4f 2 + x 2)3/2], which has its maximal value at the vertex of the parabola, i.e., at x = 0, with K(0) = 1/2f. The parabolic tubular vessels in this study were constructed by sweeping a circular cross-sectional area along a parabolic path. This path was defined as the higher curvature of the two curves obtained by intersection of the tube wall with the plane containing the entire centerline. In defining the geometry this way, the maximum curvature that may be defined, without causing the tube to intersect upon itself, is virtually unbounded. A caveat to defining the geometry in this manner is that it complicates the equation for the centerline, since a curve parallel to a parabola is, in fact, not a parabola. To maintain a total tube centerline arc length of 40 mm, for varying focal lengths, an equation describing the centerline was determined as a function of the parabolic focal length and the tube’s radius. Within the Autodesk Inventor software, a macro was written to iterate the centerline’s end points, utilizing the Newton–Rhapson method, such that a total vessel centerline length of 40 mm is maintained, for all focal lengths considered. ANSYS’ Design of Experiments feature was then used to automate: generating the desired tubular pathway, meshing the domain and numerically solving the associated fluid dynamics differential equations with FLUENT.
Rights and permissions
About this article
Cite this article
Celestin, C., Guillot, M., Ross-Ascuitto, N. et al. Computational Fluid Dynamics Characterization of Blood Flow in Central Aorta to Pulmonary Artery Connections: Importance of Shunt Angulation as a Determinant of Shear Stress-Induced Thrombosis. Pediatr Cardiol 36, 600–615 (2015). https://doi.org/10.1007/s00246-014-1055-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-014-1055-7