Abstract
We evaluated whether near-infrared spectroscopy (NIRS) measurement from the flank correlates with renal vein saturation in children undergoing cardiac catheterization. Thirty-seven patients <18 years of age were studied. A NIRS sensor was placed on the flank, and venous oxygen saturations were measured from the renal vein and the inferior vena cava (IVC). There was a strong correlation between flank NIRS values (rSO2) and renal vein saturation (r = 0.821, p = 0.002) and IVC saturation (r = 0.638, p = 0.004) in children weighing ≤ 10 kg. In children weighing > 10 kg, there was no correlation between rSO2 and renal vein saturation (r = 0.158, p = 0.57) or IVC saturation (r = –0.107, p = 0.67). Regional tissue oxygenation as measured by flank NIRS correlates well with both renal vein and IVC oxygen saturations in children weighing <10 kg undergoing cardiac catheterization, but not in larger children.
Similar content being viewed by others
Introduction
Near-infrared spectroscopy (NIRS) is a noninvasive technology that uses the different absorption spectra of oxy-hemoglobin and deoxy-hemoglobin to provide a continuous estimate of regional tissue oxygen saturation (rSO2). The most common clinical application of this technology has been in assessing cerebral oxygen saturation with a probe placed on the forehead. Studies have shown a correlation between cerebral oxygenation as measured by NIRS and jugular venous, central, and mixed venous saturations [5, 13, 14, 16, 23].
Due to cerebral autoregulation, blood flow and oxygen delivery to the brain may be preserved in low cardiac output states. Thus, cerebral NIRS values may not represent perfusion to other organs during low cardiac output. This has lead to NIRS technology being employed at multiple different sites [8, 20, 22].
NIRS monitors are now being widely used to monitor renal perfusion with a probe placed on the flank, especially in pediatric cardiac intensive care units because children undergoing cardiac surgery are at increased risk for acute renal failure due to decreased perfusion and oxygen delivery in the intraoperative and postoperative periods [1, 6, 12, 24]. Preliminary evidence presented by Hoffmann et al. suggest that renal NIRS values in this group are associated with regional ischemia and changes in kidney function [10, 11]. Although flank rSO2 has been found to weakly correlate with superior vena cava saturations in infants [14, 15], there are no published studies investigating whether these readings actually correlate with renal vein saturations.
The purpose of this study was to evaluate whether NIRS measurements from the flank correlate with renal vein saturations in children. Inferior vena cava (IVC) oxygen saturations were also measured and compared with NIRS because saturations from this location have been used as an indicator of global perfusion [9].
Materials and Methods
Study Population
Pediatric patients <18 years of age undergoing medically indicated cardiac catheterization at Arkansas Children’s Hospital were eligible for participation in this study. This study was approved by the Institutional Review Board at the University of Arkansas for Medical Sciences. Written informed consent was obtained from the legal guardians and assent from subjects >7 years.
Data Collection
In the catheterization laboratory, a NIRS probe (INVOS Cerebral/Somatic Oximeter 5100B; Somanetics Inc., Troy, MI) appropriate for weight was placed at the T10 to L2 level lateral to midline by the principal investigator before the start of the procedure. The cerebral oximeter used in this study uses NIRS technology to measure regional changes in oxygenated and deoxygenated hemoglobin in the tissue under the probe. The probe emits two different wavelengths of near-infrared light (730 and 810 nm), which correspond to the different spectral absorptions of oxygenated and deoxygenated hemoglobin. Measuring the quantity of returning light of each wavelength allows for assessment of changes in the concentrations of these molecules.
Vessel cannulation and timing of blood samples were at the discretion of the cardiologist performing the procedure. Inspired oxygen percentage delivered was based on the clinical needs of the patient. Blood samples were drawn from the left or right renal vein and the IVC below the diaphragm, but superior to the renal vein, with an end-hole catheter placed under fluoroscopic guidance. We recorded rSO2 at the time of blood sample collection. Oxygen saturations and hemoglobin level were obtained immediately after drawing the samples with a GEM OPL portable laboratory co-oximeter (Instrumentation Laboratory, Lexington, MA). Addition data collected included age, sex, diagnoses, weight, height, pulse oximetry, cardiac index as measured by Fick, and inspired oxygen percentage.
Statistical Analysis
Descriptive statistics were performed on demographic data. Subjects weighing ≤10 kg and those weighing >10 kg were analyzed separately to evaluate the effect of weight on rSO2 correlations. We compared the rSO2 values and measured venous saturations using concordance correlation coefficients, which evaluate the degree to which pairs of observations fall on the 45º line through the origin. Pearson’s correlation coefficients (a measure of precision) and bias correction factors (a measure of accuracy) were also calculated. Bland-Altman plots, which illustrate the difference versus the average of the two methods, were used to estimate the precision and bias of the study variables.
Results
Forty children were consented for study; 19 weighed ≤10 kg, and 21 weighed >10 kg. Data were not collected on 1 patient (>10 kg) due to respiratory compromise before the procedure and 2 patients (1 ≤10 kg and 1 >10 kg) who did not have blood samples drawn by the physician performing the catheterization. Mean weight was 6.2 kg (range 2.2 to 10) for subjects ≤10 kg and 18.8 kg (range 10.4 to 67) for subjects >10 kg. Mean age was 4.8 months (range 3 days to 20 months) for subjects ≤10 kg and 83 months (range 18 to 163) for subjects >10 kg. Renal vein saturations were not measured in 7 of the children weighing ≤10 kg and 4 of the children weighing >10 kg due to difficulties accessing the renal vein from the superior vena cava approach or hemodynamic instability before renal vein cannulation. Seven subjects (19%) had cyanotic heart disease with a baseline arterial saturation of <90%.
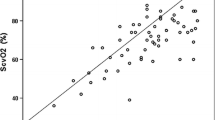
Saturations and rSO2 values for subjects weighing ≤10 kg and those weighing >10 kg are listed in Tables 1 and 2, respectively. Using Pearson’s correlation, we found a significant correlation between flank rSO2 and renal vein saturation (r = 0.821, p = 0.002) and between rSO2 and IVC saturation (r = 0.638, p = 0.004) in children ≤10 kg. Concordance correlation coefficient was 0.77 for rSO2 and renal vein saturation and 0.63 for rSO2 and IVC saturation. Bias correction factor was 0.94 for renal vein saturations and 0.99 for IVC saturations.
In children weighing >10 kg, there was no correlation between rSO2 and renal vein saturation (r = 0.158, p = 0.57) or IVC saturation (r = –0.107, p = 0.67). The concordance correlation coefficient was also low at 0.15 for rSO2 and renal vein saturation and −0.07 for rSO2 and IVC saturation. Bias correction factor was 0.94 for renal vein saturations and 0.7 for IVC saturations in children weighing >10 kg. Bland-Altman plots demonstrating the bias and precision of these measurements are shown in Fig. 1.
Discussion
Our study clearly indicates that there is a significant correlation between rSO2 as measured by flank NIRS and renal vein saturation in children weighing <10 kg. There is also a good correlation between rSO2 and IVC saturation. The NIRS measurements were noted to be both precise and accurate in this group. There was no correlation between the measured renal vein and IVC saturations and the flank rSO2 in children >10 kg. Among this group rSO2 was a low precision but highly accurate estimate of renal vein saturation, as can be seen in the Bland-Altman plots.
Children undergoing repair of congenital heart disease are at high risk of developing acute renal failure with the incidence of postoperative dialysis varying from 2.3% to >30% [3, 18, 23]. Children who develop renal failure after surgery are at much greater risk of mortality [3, 6]. Although the mechanisms for renal failure after cardiac surgery are multifactorial, diminished renal perfusion secondary to decreased myocardial function and hypotension leading to ischemic tubular necrosis is thought to be the main contributor.
The kidney receives a disproportionately high fraction of the cardiac output compared with its metabolic need; however, the local mechanisms that balance oxygen delivery and use and low oxygen tension in the outer medulla put the kidney at risk for hypoxic injury [7]. Late indicators, such as oliguria and increased creatinine, are used to assess renal perfusion but are limited because renal injury has already occurred by the time these clinical changes are noted. NIRS may provide an accurate, noninvasive, real-time measure of renal oxygenation. Interventions to correct the oxygen supply–demand balance could potentially decrease the incidence of renal dysfunction in patients at risk for decreased renal perfusion.
Few studies have reported on flank NIRS values and actual patient clinical outcomes. In children undergoing repair of coarctation of the aorta, cross-clamping of the aorta was accompanied by a large decrease in somatic oxygenation [4]. Owens et al. studied somatic oxygenation as measured by flank NIRS in infants undergoing biventricular repair [17]. NIRS values <50% for >2 h after surgery were predictive of increased creatinine and a greater incidence of acute kidney injury by 48 h. They also found that those with low flank NIRS had worse clinical outcomes, including increased lactate, more ventilator days, and greater vasoactive medication need.
Most studies using NIRS have focused on measurements of regional cerebral oxygenation, but clinicians should use caution in applying these studies to NIRS use in other locations. Oxygen use and control of blood flow differ remarkably in different body tissues. In a study using both cerebral and flank NIRS, Hanson et al. saw a significant increase in somatic NIRS values with fluid resuscitation in moderately dehydrated patients [9]. Cerebral NIRS values were greater at baseline and did not change during rehydration. During times of decreased cardiac output, sympathetic vasoconstriction will shunt blood away from vascular beds, such as mesenteric, renal, and cutaneous, to preserve blood flow to the brain. Thus, decreased somatic NIRS in the context of normal cerebral NIRS may give an earlier indication of impaired output.
Our study found a correlation between flank NIRS measurements and IVC saturations in children weighing <10 kg. Use of IVC saturation as a measure of oxygen delivery to the tissues is often performed in the intensive care unit given the technical difficulties that can be present in obtaining superior vena cava or mixed venous saturations in infants [19]. The IVC receives blood from multiple tissue beds and reflects perfusion to the lower body, and NIRS measurements from the thigh have also been correlated to IVC saturations [21]. This study suggests that the flank NIRS probe can also be helpful in assessing global perfusion noninvasively.
The difference in correlations between smaller and larger children is not unexpected given the limited sampling depth of the NIRS probe. According to the manufacturer, the NIRS readings compared with somatic tissue are reflective of oxygenation approximately 1 to 2 cm beneath the sensor (http://www.somanetics.com/our-technology/nirs-technology). Balaguru et al. measured the body wall thickness of 38 children undergoing computed tomography scans [2]. The median distance between the skin and the capsule of the kidney was 22 mm and had a wide range (6.6 to 116). As expected, this distance increased with increasing age and weight. The infrared light emitted from the NIRS probe likely did not penetrate beyond the subcutaneous tissues in our larger patients. In small children, the body wall is presumably thin enough to allow the infrared light to reach the kidney parenchyma. Using weight instead of body mass index (BMI) is a limitation of our study because BMI may provide a better indication of body wall thickness than weight. High BMI was uncommon in this patient population, so a larger sample size would be needed to stratify patients by BMI.
Although flank measurements in children weighing <10 kg correlated with IVC saturations, this was not the case in the larger children, suggesting that use of a flank probe may be limited in its utility in this population. However, NIRS and saturation measurements in this study were made at a single time point, and NIRS is often clinically used to assess changes in hemodynamics over time. A probe at this location may still be useful as an indicator of tissue perfusion trends.
In conclusion, regional tissue oxygenation measured by flank NIRS correlates well with both renal vein and IVC oxygen saturations in children weighing <10 kg who are undergoing cardiac catheterization. There is no correlation between measured venous saturations and NIRS values over the flank in children weighing >10 kg. Further investigation is needed to assess how measurement of flank NIRS values are correlated with renal function and whether interventions aimed at improving perfusion and NIRS values results in less kidney injury and improved outcomes.
References
Alwaidh M, Cooke R, Judd B (1998) Renal blood flow velocity in acute renal failure following cardiopulmonary bypass surgery. Acta Paediatr 87:644–649
Balaguru D, Bhalala U, Haghighi M, Rivera V, Norton K (2008) Abdominal CT measurements of anterior and posterior abdominal wall to create reference values for use with near-infrared spectroscopy (NIRS). Pediatric Cardiac Intensive Care Meeting, Miami, Dec 2–6, 2008
Baskin E, Saygili A, Harmanci K, Agras PI, Özdemir FN, Mercan S et al (2005) Acute renal failure and mortality after open-heart surgery in infants. Renal Fail 27:557–560
Berens RJ, Stuth EA, Robertson FA, Jaquiss RD, Hoffman GM, Troshynski TJ et al (2006) Near infrared spectroscopy monitoring during pediatric aortic coarctation repair. Pediatr Anesth 16:777–781
Bhutta AT, Ford JW, Parker JG, Prodhan P, Fontenot EE, Seib PM et al (2007) Noninvasive cerebral oximeter as a surrogate for mixed venous saturation in children. Pediatr Cardiol 28:34–41
Boigner H, Brannath W, Hermon M, Stoll E, Burda G, Trittenwein G et al (2004) Predictors of mortality at initiation of peritoneal dialysis in children after cardiac surgery. Ann Thorac Surg 77:61–65
Evans R, Gardiner B, Smith D, O’Connor PM (2008) Intrarenal oxygenation: unique challenges and the biophysical basis of homeostasis. Am J Physiol Renal Physiol 295:F1259–F1270
Fortune PM, Wagstaff M, Petros AJ (2001) Cerebro-splanchnic oxygenation ratio (CSOR) using near infrared spectroscopy may be able to predict splanchnic ischaemia is neonates. Intensive Care Med 27:1401–1407
Hanson SJ, Berens RJ, Havens PL, Kim MK, Hoffman GM (2009) Effect of volume resuscitation on regional perfusion in dehydrated pediatric patients as measured by two-site near-infrared spectroscopy. Pediatr Emerg Care 25:150–153
Hoffman G, Wider M (2008) Organ specificity of NIRS rSO2 measurements during regional ischemia in piglets. Anesthesiology 109:A272
Hoffman G, Ghanayem N, Mussatto K, Musa N (2005) Perioperative perfusion assessed by somatic NIRS predicts postoperative renal dysfunction. Anesthesiology 103:A1327
Holthe Kist-van, tot-Echten JE, Goedvolk CA, Doornaar MB, van der Vorst MMJ, Bosman-Vermeeren JM, Brand R et al (2001) Acute renal insufficiency and renal replacement therapy after pediatric cardiopulmonary bypass surgery. Pediatr Cardiol 22:321–326
Kim M, Ward D, Cartwright C, Kolano J, Chlebowski S, Henson LC (2000) Estimation of jugular venous O2 saturation from cerebral oximetry or arterial O2 saturation during isocapnic hypoxia. J Clin Monit Comput 16:191–199
Li J, Van Arsdell G, Zhang G, Cai S, Humpl T, Caldarone CA et al (2006) Assessment of the relationship between cerebral and splanchnic oxygen saturations measured by near infrared spectroscopy and direct measurements of systemic haemodynamic variables and oxygen transport after the Norwood procedure. Heart 92:1678–1685
McQuillen PS, Nishimoto MS, Bottrell CL, Fineman LD, Hamrick SE, Glidden DV et al (2007) Regional and central venous oxygen saturation monitoring following pediatric cardiac surgery: concordance and association with clinical variables. Pediatr Crit Care Med 8:154–160
Nagdyman N, Fleck T, Barth S, Abdul-Khaliq H, Stiller B, Ewert P et al (2004) Relation of cerebral tissue oxygenation index to central venous oxygen saturation in children. Intensive Care Med 30:468–471
Owens GE, King K, Gurney JG, Chapie JR (2011) Low renal oximetry correlates with acute kidney injury after infant cardiac surgery. Pediatr Cardiol 32:183–188
Pedersen KR, Hjortdal VE, Christensen S, Pedersen J, Hjortholm K, Larsen SH et al (2008) Clinical outcome in children with acute renal failure treated with peritoneal dialysis after surgery for congenital heart disease. Kidney Int 108(Suppl):S81–S86
Plötz F, van Lingen R, Bos A (1998) Venous oxygen measurements in the inferior vena cava in neonates with respiratory failure. Critical Care 2:57–60
Redlin M, Boettcher W, Huebler M, Berger F, Hetzer R, Koster A (2006) Detection of lower torso ischemia by near-infrared spectroscopy during cardiopulmonary bypass in a 6.8 kg infant with complex aortic anatomy. Ann Thorac Surg 52:323–325
Redlin M, Koster A, Heubler M, Boettcher W, Nadgyman N, Hetzer R et al (2008) Regional differences in tissue oxygenation during cardiopulmonary bypass for correction of congenital heart disease in neonates and small infants: relevance of near-infrared spectroscopy. J Thorac Cardiovasc Surg 136:962–967
Weiss M, Schulz G, Teller I, Dullenkopf A, Kolarova A, Sailers H et al (2004) Tissue oxygenation monitoring during major pediatric surgery using transcutaneous liver near infrared spectroscopy. Pediatr Anesth 14:989–995
Weiss M, Dullenkopf A, Kolarova A, Schulz G, Frey B, Baenziger O (2005) Near-infrared spectroscopic cerebral oxygenation reading in neonates and infants is associated with central venous oxygen saturation. Pediatr Anesth 15:102–109
Wernovsky G, Wypij D, Jonas RA, Mayer JE, Hanley FL, Hickey PR, Walsh AZ et al (1995) Postoperative course and hemodynamic profile after the arterial switch operation in neonates and infants. A comparison of low-flow cardiopulmonary bypass and circulatory arrest. Circulation 92:2226–2235
Acknowledgments
This study was performed at Arkansas Children’s Hospital, Little Rock, AR. Funding was received from the University of Arkansas for Medical Sciences College of Medicine Dean’s/CUMG/Research Development Fund Grant Program.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Ortmann, L.A., Fontenot, E.E., Seib, P.M. et al. Use of Near-Infrared Spectroscopy for Estimation of Renal Oxygenation in Children With Heart Disease. Pediatr Cardiol 32, 748–753 (2011). https://doi.org/10.1007/s00246-011-9960-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-011-9960-5