Abstract
The success rate of right-heart bypass surgery in patients with a functionally single ventricle (f-SV) and systemic obstruction is low. In patients with a high risk of subaortic stenosis, we performed an initial step of pulmonary artery banding (PAB) and arch reconstruction before placing a bidirectional cavopulmonary shunt (BCPS) in infants with or without Damus-Kaye-Stansel (DKS) anastomosis. We assessed the success of right-heart bypass surgery. Between October 2003 and August 2008, we performed surgery in 19 neonates (median age 5 days) with f-SV and arch obstruction. Extended aortic arch anastomosis, with or without distal arch augmentation, was performed in 10 patients, and subclavian flap aortoplasty was performed in 9 patients. The circumference of the PAB was determined as the individual patient’s body weight in kilograms plus 16.2 ± 3.7 mm. Eighteen of 19 infants (95%) underwent successful BCPS placement at a median age of 7.8 months. DKS anastomosis was performed concomitantly during BCPS placement in 11 infants in whom subaortic stenosis was morphologically suspected but not demonstrated physiologically. As our first-stage operation, arch reconstruction plus PAB provided high success rates for right-heart bypass operations. This strategy is not leading, but it is a reliable approach for progression along a Fontan pathway.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Placement of a bidirectional cavopulmonary shunt (BCPS) as a staging procedure to Fontan operation can lead to decreased ventricular volume overload in younger patients, resulting in a low mortality rate [15, 20, 22]. However, the success rate of right-heart bypass operations are low in patients with aortic obstruction and a functionally single ventricle (f-SV) [16, 18]. Odim et al. [16] and Rodefeld et al. [18]. reported the success rate of right-heart bypass operations as 60% and 64%, respectively, in patients with aortic obstruction and f-SV. Poor outcomes in this group of patients were attributed to ventricular hypertrophy caused by progressive systemic outflow tract obstruction [7, 8]. Several surgical approaches [4, 11, 16, 21] have been used for the management of patients with f-SV and aortic arch obstruction. However, the choice of procedure for the management of this group remains controversial.
Pulmonary artery banding (PAB) and aortic arch reconstruction is the traditional, simple, and less invasive approach. Because younger patients can undergo BCPS placement, we considered that PAB in the staged Fontan era should be tighter than that in the non–staged Fontan era to protect pulmonary vascular beds from high pressure loads. To circumvent the development of systemic outflow tract obstruction, we performed aggressive augmentation of a hypoplastic distal arch, before BCPS placement, in infants with or without Damus-Kaye-Stansel (DKS) anastomosis.
The aim of the present study was to assess the impact of our first-stage operation for aortic arch obstruction and single-ventricle physiology on the progression of the systemic outflow tract obstruction, protecting effects of pulmonary vascular beds and ventricular function, and the success rate of a subsequent right heart bypass operation.
Methods
All parents of patients were informed of the surgical risks as well as our staging strategy toward Fontan operation at our institute, and appropriate consent was obtained. Clinical records were reviewed to document clinical features, operative procedures, and perioperative courses. The present study was approved by the ethics committee of Kanagawa Children’s Medical Center. The investigators had full access to the data and take responsibility for its integrity. All investigators have read and agreed to the manuscript as written.
Patients
At Kanagawa Children’s Medical Center between October 2003 and August 2008, 19 neonates with f-SV and aortic arch obstruction underwent first-stage repair without cardiopulmonary bypass. Preoperative characteristics of the patients arte listed in Table 1. Patient age ranged from 0 to 43 days (median 5), and body weight ranged from 1.8 to 3.9 kg (median 2.9). All of them were diagnosed as Fontan candidates. We defined that the hypoplastic ventricle was ≤ 60% of normal ventricular end-diastolic volume using echocardiogram or cardiac catheterization. Nine patients were diagnosed with distal aortic arch hypoplasia [10]. Four patients had heterotaxy syndrome; 1 patient had Down syndrome; and 1 patient had VATER association (vertebral defects, anal atresia, tracheoesophageal fistula, and radial defects).
The morphology of the atrioventricular (AV) valve consisted of common AV valve in five patients, mitral valve in six patients, and tricuspid valve in seven patients. Atrioventricular valve regurgitation (AVVR) was assessed as mild or less in all cases. Low-oxygen therapy with nitrogen inhalation was administered preoperatively in six patients.
During the same period, 37 neonates and infants with f-SV underwent banding of the main pulmonary artery as the first stage to Fontan operation. Morphologic features of the aortic arch, the pulmonary artery, and intracardiac defects were evaluated preoperatively using echocardiogram or three-dimensional helical computed tomography. The decision of surgical approach was made on the basis of these data and the general condition of each patient. Aortic arch obstruction was suggested a right-to-left shunting across the ductus. The distal aortic arch was hypoplastic if its narrowest diameter was less than the weight of the baby in kilograms plus 1 mm [10]. Five patients with f-SV and severe subaortic or aortic valve stenosis were excluded from the study because they had successfully undergone Norwood surgery. In these five patients retrospectively, subaortic, aortic valve, or bulboventricular foramen size was <0.7 cm2/m2 per the preoperative echocardiogram measurement.
Surgical Techniques
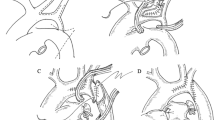
Several aortic arch reconstruction procedures were used according to morphologic characteristics, including length of the isthmus, size of the distal arch, and diameter and anatomy of the left subclavian artery. Coarctectomy plus extended aortic arch anastomosis, with or without distal arch augmentation from a median sternotomy, was performed in 10 patients, and subclavian flap aortoplasty from a lateral thoracotomy was performed in 9 patients. The distal aortic arch was augmented using the modified method reported by Amato et al. [2] and involved side-to-side anastomosis between the left common carotid artery and the distal aortic arch. A modification in our approach was that augmentation of the arch was performed as the first step, after which extended aortic arch anastomosis was performed by way of a median sternotomy.
All patients underwent main PAB after aortic arch reconstruction. A 0.6-mm thick × 3.0-mm wide expanded polytetra fluoroethylene (ePTFE) was used. At first, circumference of the band was determined as the patient’s body weight in kilograms plus 18 mm (guided by 6-0 monofilament sutures). We were careful to properly place the band, avoiding migration distally as well placement too proximally, which eliminates the risk of pulmonary valve deformation for successful performance of the pulmonary valve after the later DKS procedure. The tightness of the band was considered appropriate when the oxygen level was approximately 35 mm Hg at 60% Fio 2 during surgery. Consequently, the circumference of the band was determined as the individual patient’s body weight in kilograms plus 16.1 ± 3.8 mm (Fig. 1).
Statistical Analysis
Continuous data are expressed as mean values ± SDs or medians. Analyses were performed using SPSS software (version 11.0 J; SPSS, Chicago, IL). The probability of BCPS placement achievement rate was calculated using the Kaplan-Meier method.
Results
Postoperative Course
There was no hospital death. Additional early reoperation was required in two patients. In one patient whose distal arch hypoplasia was diagnosed as minimal before surgery, the residual pressure gradient increased to 30 mm Hg at the distal arch. Thus, we augmented the distal aortic arch on the day after the initial operation. In another patient, a restrictive atrial septal defect (ASD) and a ventricular septal defect (VSD) were discovered after the initial procedure. We enlarged the VSD and created an ASD using cardiopulmonary bypass.
Patients were deeply sedated and paralyzed during the first 12 hours of surgery. The median duration of postoperative mechanical ventilator support was 2 days (range 1 to 25). At discharge, median percutaneous oxygen saturation was 80% (range 75% to 90%). Echocardiogram at discharge showed that median pressure gradient and velocity of the main pulmonary artery at the band site were, respectively, 52 mm Hg (range 31 to 85) and 3.6 m/s (range 2.8 to 4.6); AVVR was mild or less in all patients. There was no significant stenosis of the aortic arch or the systemic outflow tract.
Additional late operation was required in one patient who underwent placement of a right modified Blalock-Taussig shunt because of distortion and poor growth of the right pulmonary artery caused by band migration. Home oxygen therapy was induced before BCPS placement to decrease pulmonary vascular resistance in one patient who had Down syndrome.
Success of Right Heart Bypass
One patient could not undergo placement of a BCPS because she died of heart failure with sepsis. The subaortic space was morphologically stenotic in 10 patients on angiography (Fig. 2) or echocardiogram before BCPS placement, although no significant pressure gradient was demonstrated in any patient. In these patients, concomitant DKS was performed at the time of BCPS placement (Table 1).
Angiography of patients in whom concomitant DKS was performed at BCPS placement surgery. a Case no. 14. b Case no. 17. The subaortic space was morphologically stenotic, although no significant pressure gradient was demonstrated in these patients. Asc Ao ascending aorta; BVF bulboventricular foramen; MPA main pulmonary artery
Cardiac catheterization showed pulmonary-to-systemic flow ratio (Qp/Qs) 1.1 ± 0.5; pulmonary arterial pressure 16 ± 6 mm Hg; arterial oxygen saturation 82% ± 6%; pulmonary artery (PA) index 294 ± 111 cm2/m2; and pulmonary resistance 1.9 ± 0.6 U × m2. AVVR remained mild or less as shown by both ventriculography and echocardiography. Ventricular function was maintained at a normal range with ejection fraction 63% ± 14%, ventricular end-diastolic volume 165% ± 48% of normal, and ventricular end-diastolic pressure 7 ± 3 mm Hg (Table 2). There was no significant pressure gradient between the proximal aortic arch and descending aorta in any patient.
All survivors underwent successful BCPS placement at a median age of 7.8 months (range 3.2 to 23.7). BCPS placement achievement rate was 78% at 12 months of age (Fig. 3). Concomitant procedures with BCPS placement included patch plasty of the central pulmonary artery in two patients, ASD enlargement or creation in five patients, VSD enlargement in one patient, and tricuspid valve plasty in two patients. One patient underwent concomitant patch enlargement of the aortic arch with BCPS placement at the stenotic site of the distal arch (Table 1). Two patients died before Fontan operation because of heart failure or mediastinitis.
At a follow-up interval of 5.9 months to 4.8 years (median 27.0 months), Fontan operation with an extracardiac conduit was performed in nine patients ranging in age from 15.3 to 36.5 months (median 24.6), and the others were awaiting Fontan surgery in good hemodynamic states.
Discussion
In the present study, we demonstrated that PAB with aortic arch reconstruction, without cardiopulmonary bypass, in patients with f-SV and aortic arch obstruction produced acceptable mid-term outcomes. The potential hazards of systemic ventricular outflow tract obstruction were successfully circumvented by the concomitant procedure of DKS with BCPS placement in infancy. Results demonstrated that this strategy could prevent an f-SV from volume overload and protect the pulmonary vascular beds from vascular obstructive disease. Thus, it is likely that the treatment of f-SV and arch obstruction contributed to the high success rate of the right-heart bypass operation.
PAB and Systemic Outflow Obstruction
Patients with f-SV and aortic arch obstruction have a large pulmonary artery and luxuriant flow. Early surgical efforts should address preservation of the pulmonary vascular beds by PAB and repair of the aortic arch obstruction. Because younger patients can tolerate BCPS placement at even 2 months of age [3], we considered that PAB in the staged Fontan era can be tighter than that in the non–staged Fontan era. A tighter PAB may prevent volume overload, protect the pulmonary vascular beds and ventricular function, and improve the success rate of BCPS placement as well as final completion of the staged Fontan.
PAB is thought to accelerate the systemic ventricular outflow tract obstruction at the VSD or bulboventricular foramen in the subaortic area secondary to the effect of ventricular volume unloading [7, 8]. Franklin et al. [7] reported that the outcome after treatment of tricuspid atresia and double-inlet ventricle with a discordant ventriculoarterial connection after PAB was significantly worse in patients with an associated aortic arch obstruction compared with those without arch obstruction. In that study, all such patients developed subaortic stenosis or died by 3 years of age. Freedom et al. [8] demonstrated a high incidence of systemic outflow tract stenosis in patients with univentricular heart after PAB; 31 of 43 patients (72%) developed systemic outflow tract stenosis at a mean age of 2.52 years. In contrast, Jensen et al. [9] suggested that in a high-risk group of patients, effective PAB as an initial step, with subsequent intervention for subaortic obstruction, resulted in acceptable pre-Fontan hemodynamic parameters. Webber et al. [23] also performed PAB in infants with a double-inlet left ventricle (DILV) and transposition of the great vessels in the absence of severe subaortic stenosis and recommended early relief of subaortic stenosis combined with BCPS placement in infancy.
Repair of Distal Aortic Arch Hypoplasia
Treatment of the hypoplastic distal aortic arch has been controversial [5, 14, 17, 19, 24, 25], with some investigators suggesting that it is unnecessary to repair the hypoplastic arch because it would grow with time [5, 19]. However, there should be reasonable evidence that the hypoplastic arch is repaired at the time of coarctation repair. Qu et al. [17] reported negative correlations between the outer diameter of distal arch and postoperative pressure gradient and demonstrated that a distal arch outer diameter of 3.9 mm is needed for repair. In addition, Machii and Becker [14] examined autopsy specimens of hypoplastic aortic arch and found fewer α-actin–positive cells, which is suggestive of diminished growth potential, and they encouraged early coarctectomy and repair of hypoplastic distal arch from a morphologic point of view. In patients with f-SV, distal arch augmentation may be useful in view of circumventing the systemic ventricular outflow tract obstruction because of decreased ventricular afterload.
With regard to methods of augmenting the distal arch aortic arch, several techniques—including left carotid artery flap [1], free flap of the subclavian artery [12], and resection with extended aortic arch anastomosis [6, 13]—have been reported. Although the method used to enlarge the distal aortic arch in the present study followed that by Amato et al. [2], we introduced several important and beneficial amendments. First, the lower part of the body was perfused through a patent ductus during augmentation of the distal aortic arch. Second, during augmentation of the distal aortic arch the proximal aortic clamp was properly applied with the front view. Third, a pulmonary artery band was properly placed so as not to impair pulmonary valve function.
Alternative Approaches
Other surgical approaches have been described [4, 21]. These include the Norwood- or DKS-type operations. These early aggressive approaches have demonstrated excellent mid-term outcomes according to the reports of several groups. As mentioned previously (see Patients section), we performed Norwood surgery in some patients with successful results. Although the Norwood- or DKS-type surgical approach is useful, we do not consider it a priority approach for patients with f-SV and aortic arch obstruction. The main drawback to this approach is the complexity of using cardiopulmonary bypass in a neonate, which may carry the risk of retardation. In addition, patients are exposed to the risks of shunt thrombosis, pulmonary overcirculation with systemic hypoperfusion, and low diastolic blood pressure with resulting coronary ischemia.
Conclusion
The present study demonstrated a staged surgical strategy for the treatment of patients with f-SV with unobstructed pulmonary blood flow and aortic arch obstruction. In patients with a high probability of developing later systemic ventricular outflow tract obstruction, we also applied arch reconstruction and PAB and, subsequently, BCPS placement in infants with DKS anastomosis if morphologically indicated. Our first-stage operation for treatment of patients with f-SV and arch obstruction achieved high success rates for right-heart bypass surgery. This strategy is not a leading one, but it is a reliable approach for progression along a Fontan pathway.
References
Allen RG, Maria-Garcia J, Nayek G (1980) Methods of management and results following surgery for coarctation of the aorta in infancy. J Pediatr Surg 15:953–960
Amato JJ, Rheinlander HF, Cleveland RJ (1977) A method of enlarging the distal transverse arch in infants with hypoplasia and coarctation of the aorta. Ann Thorac Surg 23:261–263
Bradley SM, Mosca RS, Hennein HA et al (1996) Bidirectional superior cavopulmonary connection in young infants. Circulation 94:II5–II11
Bradley SM, Simsic JM, Atz AM, Dorman BH (2002) The infant with single ventricle and excessive pulmonary blood flow: results of a strategy of pulmonary artery division and shunt. Ann Thorac Surg 74:805–810; discussion 810
Brouwer MH, Cromme-Dijkhuis AH, Ebels T, Eijgelaar A (1992) Growth of the hypoplastic aortic arch after simple coarctation resection and end-to-end anastomosis. J Thorac Cardiovasc Surg 104:426–433
Elliott MJ (1987) Coarctation of the aorta with arch hypoplasia: improvements on a new technique. Ann Thorac Surg 44:321–323
Franklin RC, Sullivan ID, Anderson RH, Shinebourne EA, Deanfield JE (1990) Is banding of the pulmonary trunk obsolete for infants with tricuspid atresia and double inlet ventricle with a discordant ventriculoarterial connection? Role of aortic arch obstruction and subaortic stenosis. J Am Coll Cardiol 16:1455–1464
Freedom RM, Benson LN, Smallhorn JF et al (1986) Subaortic stenosis, the univentricular heart, and banding of the pulmonary artery: an analysis of the courses of 43 patients with univentricular heart palliated by pulmonary artery banding. Circulation 73:758–764
Jensen RA Jr, Williams RG, Laks H, Drinkwater D, Kaplan S (1996) Usefulness of banding of the pulmonary trunk with single ventricle physiology at risk for subaortic obstruction. Am J Cardiol 77:1089–1093
Karl TR, Sano S, Brawn W, Mee RB (1992) Repair of hypoplastic or interrupted aortic arch via sternotomy. J Thorac Cardiovasc Surg 104:688–695
Kouchouskos NT, Blackstone EH, Doty DB, Hanley FL, Karp RB (2003) Tricuspid atresia and management of single-ventricular physiology. In: Kirklin JW, Barratt-Boys BG (eds) Cardiac surgery. Philadelphia, PA, Churchill Livingstone, p 1124
Kubota H, Camilleri L, Legault B et al (1998) Surgical correction of the hypoplastic aortic arch by the subclavian free flap method in the neonate. J Thorac Cardiovasc Surg 116:519–521
Lansman S, Shapiro AJ, Schiller MS et al (1986) Extended aortic arch anastomosis for repair of coarctation in infancy. Circulation 74:I37–I41
Machii M, Becker AE (1997) Hypoplastic aortic arch morphology pertinent to growth after surgical correction of aortic coarctation. Ann Thorac Surg 64:516–520
Masuda M, Kado H, Shiokawa Y et al (1998) Clinical results of the staged Fontan procedure in high-risk patients. Ann Thorac Surg 65:1721–1725
Odim JN, Laks H, Drinkwater DC Jr et al (1999) Staged surgical approach to neonates with aortic obstruction and single-ventricle physiology. Ann Thorac Surg 68:962–968; discussion 968
Qu R, Yokota M, Kitano M et al (1990) Surgical indication for aortic arch hypoplasia in infants. J Cardiovasc Surg (Torino) 31:796–800
Rodefeld MD, Ruzmetov M, Schamberger MS et al (2005) Staged surgical repair of functional single ventricle in infants with unobstructed pulmonary blood flow. Eur J Cardiothorac Surg 27:949–955
Siewers RD, Ettedgui J, Pahl E, Tallman T, del Nido PJ (1991) Coarctation and hypoplasia of the aortic arch: Will the arch grow? Ann Thorac Surg 52:608–614; discussion 613–614
Tanoue Y, Sese A, Ueno Y, Joh K, Hijii T (2001) Bidirectional Glenn procedure improves the mechanical efficiency of a total cavopulmonary connection in high-risk Fontan candidates. Circulation 103:2176–2180
Tchervenkov CI, Shum-Tim D, Beland MJ, Jutras L, Platt R (2001) Single ventricle with systemic obstruction in early life: comparison of initial pulmonary artery banding versus the Norwood operation. Eur J Cardiothorac Surg 19:671–677
Trusler GA, Williams WG, Cohen AJ et al (1990) William Glenn lecture. The cavopulmonary shunt. Evolution of a concept. Circulation 82:IV131–IV138
Webber SA, LeBlanc JG, Keeton BR et al (1995) Pulmonary artery banding is not contraindicated in double inlet left ventricle with transposition and aortic arch obstruction. Eur J Cardiothorac Surg 9:515–520
Wood AE, Javadpour H, Duff D, Oslizlok P, Walsh K (2004) Is extended arch aortoplasty the operation of choice for infant aortic coarctation? Results of 15 years’ experience in 181 patients. Ann Thorac Surg 77:1353–1358; discussion 1357–1358
Wright GE, Nowak CA, Goldberg CS et al (2005) Extended resection and end-to-end anastomosis for aortic coarctation in infants: Results of a tailored surgical approach. Ann Thorac Surg 80:1453–1459
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kajihara, N., Asou, T., Takeda, Y. et al. Staged Surgical Approach in Neonates with a Functionally Single Ventricle and Arch Obstruction: Pulmonary Artery Banding and Aortic Arch Reconstruction Before Placement of a Bidirectional Cavopulmonary Shunt in Infants. Pediatr Cardiol 31, 33–39 (2010). https://doi.org/10.1007/s00246-009-9540-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-009-9540-0