Abstract
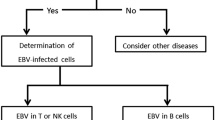
This study aimed to assess the outcome of cardiovascular diseases for patients with chronic active Epstein–Barr virus infection (CAEBV). The study enrolled 15 patients (7 boys and 8 girls) who fulfilled the diagnostic criteria for CAEBV, including 10 patients with T-cell type and 3 patients with natural killer (NK)-cell type. The median age at the CAEBV onset was 6.3 years (range, 1.2–17.8 years). Regular cardiologic studies were performed during the median follow-up period of 8 years (range, 2–20 years). Nine patients (60%) had cardiac diseases including coronary artery lesion (CAL) (n = 4, 44%), decreased left ventricular ejection fraction and pericardial effusion in (n = 3, 33%), complete atrioventricular block (n = 1), and sudden arrest (n = 1). The frequency of fever (78%, p = 0.04) or cytopenias (100%, p = 0.01), as the major symptom among patients with cardiac complications, was higher than among those without complications. The median time from disease onset to detection of CAL was 3.4 years (range, 1.8–8.6 years). The mean z-score increased to 3.98. Seven patients (78%) with cardiac complications died of disease progression, hematopoietic stem cell transplantation–related events, or both. In two patients, CAL regressed after allogeneic cord blood transplantation. Among CAEBV patients, CAL was the most common cardiac complication and could not be controlled without the eradication of EBV-infected T- and NK-cells.

Similar content being viewed by others
Abbreviations
- BMT:
-
Bone marrow transplantation
- CAL:
-
Coronary artery lesion
- CBT:
-
Cord blood transplantation
- CAEBV:
-
Chronic active Epstein–Barr virus infection
- HSCT:
-
Hematopoietic stem cell transplantation
- LVEF:
-
Left ventricular ejection fraction
References
Brown TJ, Crawford SE, Cornwall ML, Garcia F, Shulman ST, Rowley AH (2001) CD8 T lymphocytes and macrophages infiltrate coronary artery aneurysms in acute Kawasaki disease. J Infect Dis 184:940–943
Burns JC, Glodé MP (2004) Kawasaki syndrome. Lancet 364:533–544
Chuang HC, Lay JD, Chuang SE et al (2007) Epstein–Barr virus (EBV) latent membrane protein-1 downregulates tumor necrosis factor-alpha (TNF-alpha) receptor-1 and confers resistance to TNF-alpha-induced apoptosis in T-cells: implication for the progression to T-cell lymphoma in EBV-associated hemophagocytic syndrome. Am J Pathol 170:1607–1617
Culora GA, Moore IE (1997) Kawasaki disease, Epstein–Barr virus, and coronary artery aneurysms. J Clin Pathol 50:161–163
De Zorzi A, Colan SD, Gauvreau K et al (1998) Coronary artery dimensions may be misclassified as normal in Kawasaki disease. J Pediatr 133:254–258
Fujiwara M, Shimozono H, Ono H et al (2003) Polyclonal proliferation of lymphocytes containing the Epstein–Barr virus genome in a patient dying of myocarditis in chronic active Epstein–Barr virus infection. J Pediatr Hematol Oncol 25:85–88
Gallot G, Hamidou MA, Clémenceau B et al (2006) T-cell repertoire and Epstein–Barr virus-specific T-cell response in chronic active Epstein–Barr virus infection: a case study. Clin Immunol 119:79–86
Hauptmann S, Meru N, Schewe C et al (2001) Fatal atypical T-cell proliferation associated with Epstein–Barr virus infection. Br J Haematol 112:377–380
Ishimura M, Ohga S, Nomura A et al (2005) Successful umbilical cord blood transplantation for severe chronic Epstein–Barr virus infection after the double failure of hematopoietic stem cell transplantation. Am J Hemtol 80:207–212
Iwatsuki K, Satoh M, Yamamoto T et al (2006) Pathogenic link between hydroa vacciniforme and Epstein–Barr virus–associated hematologic disorders. Arch Dermatol 142:587–595
Jeanette JC (2006) Implication for pathogenesis of patterns of injury in small- and medium-sized vessel vasculitis. Cleveland Clin J Med 69:SII-33–SII-38
Kanamaru H, Sato Y, Takayama T et al (2005) Assessment of coronary artery abnormalities by multislice spiral computed tomography in adolescents and young adults with Kawasaki disease. Am J Cardiol 95:522–525
Kanno H, Onodera H, Endo M et al (2005) Vascular lesion in a patient of chronic active Epstein–Barr virus infection with hypersensitivity to mosquito bites: vasculitis induced by mosquito bite with the infiltration of nonneoplastic Epstein–Barr virus-positive cells and subsequent development of natural killer/T-cell lymphoma with angiodestruction. Human Pathol 36:212–218
Kasahara Y, Yachie A, Takei K et al (2001) Differential cellular targets of Epstein–Barr virus (EBV) infection between acute EBV-associated hemophagocytic lymphohistiocytosis and chronic active EBV infection. Blood 98:1882–1888
Kikuta H, Taguchi Y, Tomizawa K et al (1988) Epstein–Barr virus genome-positive T lymphocytes in a boy with chronic active EBV infection associated with Kawasaki-like disease. Nature 333:455–457
Kikuta H, Sakiyama Y, Matsumoto S et al (1993) Detection of Epstein–Barr virus DNA in cardiac and aortic tissue from chronic, active Epstein–Barr virus infection associated with Kawasaki-disease like coronary artery aneurysms. J Pediatr 123:90–92
Kimura H, Hoshino Y, Kanegane H et al (2001) Clinical and virologic characteristics of chronic active Epstein–Barr virus infection. Blood 98:280–286
Kimura H, Morishima T, Kanegane H et al (2003) Prognostic factors for chronic active Epstein–Barr virus infection. J Infect Dis 187:527–533
Kurotobi S, Nagai T, Kawakami N, Sano T (2002) Coronary diameter in normal infants, children, and patients with Kawasaki disease. Pediatr Int 44:1–4
Lay JD, Chuang SE, Rowe M, Su IJ (2003) Epstein–Barr virus latent membrane protein-1 mediates upregulation of tumor necrosis factor-alpha in EBV-infected T-cells: implications for the pathogenesis of hemophagocytic syndrome. J Biomed Sci 10:146–155
Murakami K, Ohsawa M, Hu SX et al (1998) Large-vessel arteritis associated with chronic active Epstein–Barr virus infection. Arthritis Rheum 41:369–373
Muso E, Fujiwara H, Yoshida H et al (1993) Epstein–Barr virus genome-positive tubulointerstitial nephritis associated with Kawasaki disease-like coronary aneurysms. Clin Nephrol 40:7–15
Nakagawa A, Ito M, Iwaki T et al (1996) Chronic active Epstein–Barr virus infection with giant coronary aneurysms. Am J Clin Pathol 105:733–736
Nakagawa A, Ito M, Saga S (2002) Fatal cytotoxic T-cell proliferation in chronic active Epstein–Barr virus infection in childhood. Am J Clin Pathol 117:283–290
Newberger JW, Takahashi M, Gerber MA et al (2004) Diagnosis, treatment, and long-term management of Kawasaki disease: a statement for health professionals from the Committee on Rheumatic Fever, Endocarditis and Kawasaki disease, Council on Cardiovascular Disease in the Young, American Heart Association. Circulation 110:2747–2761
Okano M, Kawa K, Kimura H et al (2005) Proposed guidelines for diagnosing chronic active Epstein–Barr virus infection. Am J Hematol 80:64–69
Ohga S, Nomura A, Takada H et al (2001) Epstein–Barr virus (EBV) load and cytokine gene expression in activated T-cells of chronic active EBV infection. J Infect Dis 183:1–7
Ohga S, Nomura A, Takada H et al (2004) Dominant expression of interleukin-10 and transforming growth factor-β genes in activated T-cells of chronic active Epstein–Barr virus infection. J Med Virol 74:449–458
Ohshima K, Suzumiya J, Sugihara M et al (1998) Clinicopathological study of severe chronic active Epstein–Barr virus infection that developed in association with lymphoproliferative disorder and/or hemophagocytic syndrome. Pathol Int 48:934–943
Ottaviani G, Matturri L, Rossi L, Jones D (2003) Sudden death due to lymphomatous infiltration of the cardiac conduction system. Cardiovasc Pathol 12:77–81
Sato Y, Tsuboi T, Mikami T et al (2006) Chronic active Epstein–Barr virus infection with dilatation of the Valsalva sinus. Pediatr Int 48:643–645
Satoh M, Oyama N, Akiba H et al (2002) Hypersensitivity to mosquito bites with natural-killer cell lymphocytosis: the possible implication of Epstein–Barr virus reactivation. Eur J Dermatol 12:381–384
Takano H, Nakagawa K, Ishio N et al (2008) Active myocarditis in a patient with chronic active Epstein–Barr virus infection. Int J Cardiol 130:e11–e13
Teruya-Feldstein J, Jaffe ES, Burd JP et al (1997) The role of Mig, the monokine induced by interferon-γ, and IP-10, the interferon-γ-inducible protein-10, in tissue necrosis and vascular damage associated with Epstein–Barr virus-positive lymphoproliferative disease. Blood 90:4099–4105
Toubo T, Ohga S, Takada H et al (2006) Rheumatic fever–mimicking carditis as a first presentation of chronic active Epstein–Barr virus infection. Acta Pediatr 95:614–621
Tsuda E, Kamiya T, Kimura K et al (2002) Coronary artery dilatation exceeding 4.0 mm during acute Kawasaki disease predicts a high probability of subsequent late intima-medial thickening. Pediatr Cardiol 23:9–14
Acknowledgments
This work was supported by a Grant-in-Aid for Scientific Research (C) to Shouichi Ohga and a grant by the Ministry of Education, Culture, Sports, Science and Technology of Japan to Jun Muneuchi. We thank Dr. Brian Thomas Quinn (Associate Professor, Department of Linguistic Environment, Faculty of Languages and Cultures, Kyushu University) for kindly correcting the manuscript.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Muneuchi, J., Ohga, S., Ishimura, M. et al. Cardiovascular Complications Associated with Chronic Active Epstein–Barr Virus Infection. Pediatr Cardiol 30, 274–281 (2009). https://doi.org/10.1007/s00246-008-9343-8
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00246-008-9343-8