Abstract
Many organisms appear to exhibit adaptive cost–benefit behaviors that balance foraging, safety, and pollution avoidance. However, what if the cognitive facilities needed to make decisions are compromised by industrial pollutants? Are the resulting decisions altered? Similarly, does exposure to kairomones from predators alter an organism’s ability to avoid toxicants? Furthermore, how long an exposure is necessary: A few minutes, hours, or even a lifetime? We wondered if there was an interaction between the ability to respond to a predatory event and the ability to avoid heavy metals.





Similar content being viewed by others
References
Amiard-Triquet C (2009) Behavioral disturbances: the missing link between sub-organismal and supra-organismal responses to stress? Prospects based on aquatic research. Human Ecol Risk Assess 15:87–110
Amiard-Triquet C, Rainbows PS (2011) Tolerance and the trophic transfer of contaminants. In: Amiard-Triquet C, Rainbows PS, Romeo M (eds) Tolerance to Environmental Contaminants. CRC Press, Boca Raton, pp 299–332
Atema J, Stenzler D (1977) Alarm substance of the marine mud snail, Nassarius obsoletus: biological characterization and possible evolution. J Chem Ecol 3:173–187
Bernot RJ, Kennedy EE, Lamberti GA (2005) Effects of ionic liquids on the survival, movement, and feeding behavior of the freshwater snail, Physa acuta. Environ Toxicol Chem 24:1759–1760
Calabrese EJ, Baldwin LA (2001) Hormesis: U-shaped dose response and their centrality in toxicology. Trends Pharmacol Sci 22:285–291
Campbell JK, Audet D, Kern JW, Reyes M, McDonald LL (1999) Metal contamination of palustrine and lacustrine habitats in the Coeur d’Alene Basin, Idaho. Final Report, United States Fish and Wildlife Service
Clements WH (1999) Metal tolerance and predator-prey interactions in benthic macroinvertebrate stream communities. Ecol Appl 9:1073–1084
Covich AP, Crowl TA, Alexander JE, Vaughn CC (1994) Predator-avoidance responses in freshwater decapod-gastropod interactions mediated by chemical stimuli. J North Am Benthol Soc 13:283–290
Croteau MN, Luoma SN, Pellet B (2007) Determining metal assimilation efficiency in aquatic invertebrates using enriched stable metal isotope tracers. Aquat Toxicol 83:116–125
Cypser JR, Johnson TE (2002) Multiple stressors in Caenorhabditis elegans induce stress hormesis and extended longevity. J Gerontol A Biol Sci Med Sci 57:B109–B114
Davis JM, Svendsgaard DJ (1990) U-shaped dose–response curves: their occurrence and implications for risk assessment. J Toxicol Environ Health 30:71–83
Dickey BF, McCarthy TM (2007) Predator and prey interactions between crayfish (Orconectes juvenilis) and snails (Physa gyrina) are affected by spatial scale and chemical cues. Invert Biol 126:57–66
Ellis MM (1940) Pollution of the Coeur d’ Alene river and adjacent waters by mine wastes. United States Bureau of Fisheries Special Science Report
Farag AM, Woodward DF, Goldstein JN, Brumbaugh W, Meyer JS (1998) Concentrations of metals associated with mining waste in sediments, biofilm, benthic macroinvertebrates, and fish from the Coeur d’Alene River Basin, Idaho. Arch Environ Contam Toxicol 34:119–127
Ferrari MCO, Wisenden BD, Chivers DP (2010) Chemical ecology of predator–prey interactions in aquatic ecosystems: a review and prospectus. Can J Zool 88:698–724
Fraker ME, Hu F, Cuddapah V, McCollum SA, Relyea RA, Hempel J et al (2009) Characterization of an alarm pheromone secreted by amphibian tadpoles that induces behavioral inhibition and suppression of the neuroendocrine stress axis. Horm Behav 55:520–529
Gearheart RA, Ridolfi CA, Miller CE, Claassen V, Thrush W (1999) Restoration alternatives plan for the Coeur d’Alene Basin natural resource damage assessment. Prepared for the Natural Resources Trustees
Golding LA, Timperley MH, Evans CW (1997) Non-lethal responses of the freshwater snail potamopyrgus antipodarum to dissolved arsenic. Earth Environ Sci 47:239–254
Hoang T, Rogevich E, Rand G, Frakes R (2008) Copper uptake and depuration by juvenile and adult Florida apple snails (Pomacea paludosa). Ecotoxicology 17:605–615
Hunter RD (1975) Growth, fecundity, and bioenergetics in three populations of Lymnaea palustris in upstate New York. Ecology 56:50–63
Jacobsen HP, Stabell OB (2004) Antipredator behaviour mediated by chemical cues: the role of conspecific alarm signaling and predator labeling in the avoidance response of a marine gastropod. Oikos 104:43–50
Johnston BR, Molis M, Scrosati RA (2012) Predator chemical cues affect prey feeding activity differently in juveniles and adults. Can J Zool 90:128–132
Kavaliers M (1988) Brief exposure to a natural predator, the short-tailed weasel, induces benzodiazepine-sensitive analgesia in white-footed mice. Physiol Behav 43:187–193
Lefcort H, Thomson SM, Cowles EE, Harowicz HL, Livaudais BM, Roberts WE et al (1999) Ramification of predator avoidance: predator and heavy metal mediated competition between tadpoles and snails. Ecol Appl 9:1477–1489
Lefcort H, Ammann E, Eiger SM (2000) Antipredatory behavior as an index of heavy-metal pollution? A test using snails and caddisflies. Arch Environ Contam Toxicol 38:311–316
Lefcort H, Aguon MQ, Bond KA, Chapman KR, Chaquette R, Clark J et al (2002) Indirect effects of heavy metals on parasites may cause shifts in snail species compositions. Arch Environ Contam Toxicol 43:34–41
Lefcort H, Abbott DP, Cleary DA, Howell E, Keller NC, Smith MM (2004) Aquatic snails from mining sites have evolved to detect and avoid heavy metals. Arch Environ Contam Toxicol 46:478–484
Lefcort H, Freedman Z, House S, Pendleton M (2008) Hormetic effects of heavy metals in aquatic snails: is a little bit of pollution good? Ecohealth 5:10–17
Lefcort H, Vancura J, Lider E (2010) 75 Years after mining ends stream insect diversity is still affected by heavy metals. Ecotoxicology 19:1416–1425
Lefcort H, Humphries A, Tordillos K, Vancura J, Wood S (2012) Aquatic snails detect and avoid both heavy metals and fright odor on sand substrates. In: Hamalainen EM, Jarvinen S (eds) Snails: biology, ecology and conservation. Nova Science Publishing, Happauge
McClosky JT, Newman MC (1995) Sediment preference in the Asiatic clam (Corbicula fluminea) and viviparid snail (Campeloma decisum) as a response to low-level metal and metalloid contamination. Arch Environ Contam Toxicol 28:195–202
Mesa MG (1994) Effects of multiple acute stressors on the predator avoidance ability and physiology of juvenile Chinook salmon. Trans Am Fish Soc 123:786–793
Neufeld J (1987) A summary of heavy metal contamination in the lower Coeur d’Alene river valley with particular reference to the Coeur d’Alene river wildlife management area. Idaho Department of Fish and Game publication, Coeur d’Alene, pp 1–37
Rabe FW, Bauer SB (1977) Heavy metals in lakes of the Coeur d’Alene River Valley, Idaho. Northwest Sci 51:183–197
Ridolfi Engineering and Associates Inc (1993) Confirmation of exposure of natural resources to hazardous substances in the Coeur d’Alene basin of northern Idaho. Ridolfi Engineering and Associates Inc. Publications, Seattle
Rózsa KS, Salánki J (1990) Heavy metals regulate physiological and behavioral events by modulating ion channels in neuronal membranes of molluscs. J Environ Monit Assess 14:363–375
Schäfers C, Klöppel H, Takahashi Y (2007) Zooplankton avoidance behavior following spray drift exposure to fenpyroximate. Human Ecol Risk Assess 13:527–534
Sih A, Bolnick DI, Luttbeg B, Orrock JL, Peacor SD, Pintor LM et al (2010) Predator–prey naïveté, antipredator behavior, and the ecology of predator invasions. Oikos 119:610–621
von Frisch K (1938) Zur psychologie des fisch-schwarmes. Naturwissenschaften 37:601–606
Weis JS, Smith G, Zhou T (2001) Effects of contaminants on behavior. Biochemical mechanisms and ecological consequences. Biosciences 51:209–217
Weis JS, Bergey L, Reichmuth J, Candelmo A (2011) Living in a contaminated estuary: behavioral changes and ecological consequences for five species. Biosciences 61:375–385
Wisenden BD, Pollock MS, Tremaine RJ, Webb JM, Wismer ME, Chivers DP (2003) Synergistic interactions between chemical alarm cues and the presence of conspecific and heterospecific fish shoals. Behav Ecol Sociobiol 54:485–490
Acknowledgments
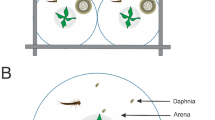
We thank John Shea for graciously providing us with Planorbidae snails. We also thank Joey Haydock and two anonymous reviewers for critically reading and improving an earlier draft of this manuscript. We are grateful to Beth Sobba for creating Fig. 1. This study was funded by a grant by the Gonzaga Summer Research Program and The Merck Foundation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lefcort, H., Wehner, E.A. & Cocco, P.L. Pre-Exposure to Heavy Metal Pollution and the Odor of Predation Decrease the Ability of Snails to Avoid Stressors. Arch Environ Contam Toxicol 64, 273–280 (2013). https://doi.org/10.1007/s00244-012-9821-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00244-012-9821-0