Abstract
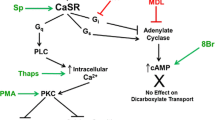
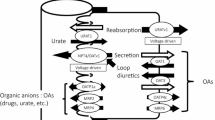
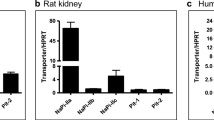
Urinary citrate is an important inhibitor of calcium-stone formation. Most of the citrate reabsorption in the proximal tubule is thought to occur via a dicarboxylate transporter NaDC1 located in the apical membrane. OK cells, an established opossum kidney proximal tubule cell line, transport citrate but the characteristics change with extracellular calcium such that low calcium solutions stimulate total citrate transport as well as increase the apparent affinity for transport. The present studies address several fundamental properties of this novel process: the polarity of the transport process, the location of the calcium-sensitivity and whether NaDC1 is present in OK cells. OK cells grown on permeable supports exhibited apical >basolateral citrate transport. Apical transport of both citrate and succinate was sensitive to extracellular calcium whereas basolateral transport was not. Apical calcium, rather than basolateral, was the predominant determinant of changes in transport. Also 2,3-dimethylsuccinate, previously identified as an inhibitor of basolateral dicarboxylate transport, inhibited apical citrate uptake. Although the calcium-sensitive transport process in OK cells is functionally not typical NaDC1, NaDC1 is present in OK cells by Western blot and PCR. By immunolocalization studies, NaDC1 was predominantly located in discrete apical membrane or subapical areas. However, by biotinylation, apical NaDC1 decreases in the apical membrane with lowering calcium. In sum, OK cells express a calcium-sensitive/regulated dicarboxylate process at the apical membrane which responds to variations in apical calcium. Despite the functional differences of this process compared to NaDC1, NaDC1 is present in these cells, but predominantly in subapical vesicles.









Similar content being viewed by others
Notes
OKP cells are a subclone of the original cell line of OK cells [14].
References
Pak CY (1987) Citrate and renal calculi. Miner Electrolyte Metab 13(4):257–266
Pajor AM, Sun N (1996) Functional differences between rabbit and human Na(+)-dicarboxylate cotransporters, NaDC-1 and hNaDC-1. Am J Physiol 271(5 Pt 2):F1093–F1099
Hering-Smith KS, Gambala CT, Hamm LL (2000) Citrate and succinate transport in proximal tubule cells. Am J Physiol 278(3):F492–F498
Hering-Smith KS, Schiro FR, Pajor AM, Hamm LL (2011) Calcium sensitivity of dicarboxylate transport in cultured proximal tubule cells. Am J Physiol Renal Physiol 300(2):F425–F432
Taylor EN, Curhan GC (2009) Demographic, dietary, and urinary factors and 24-h urinary calcium excretion. Clin J Am Soc Nephrol 4(12):1980–1987
Law D, Hering-Smith KS, Hamm LL (1992) Citrate transport in proximal cell line. Am J Physiol 263(1 Pt 1):C220–C225
Smith RM, Martell AE (1989) Critical stability constants. Plenum Press, New York
Burckhardt BC, Burckhardt G (2003) Transport of organic anions across the basolateral membrane of proximal tubule cells. Rev Physiol Biochem Pharmacol 146:95–158
Pajor AM (2000) Molecular properties of sodium/dicarboxylate cotransporters. J Membr Biol 175(1):1–8
Aruga S, Pajor AM, Nakamura K, Liu L, Moe OW, Preisig PA et al (2004) OKP cells express the Na-dicarboxylate cotransporter NaDC-1. Am J Physiol Cell Physiol 287(1):C64–C72
Wright SH, Wunz TM (1987) Succinate and citrate transport in renal basolateral and brush-border membranes. Am J Physiol 253(3 Pt 2):F432–F439
Jorgensen KE, Kragh-Hansen U, Roigaard-Petersen H, Sheikh MI (1983) Citrate uptake by basolateral and luminal membrane vesicles from rabbit kidney cortex. Am J Physiol 244(6):F686–F695
Barac-Nieto M (1984) Effects of pH, calcium, and succinate on sodium citrate cotransport in renal microvilli. Am J Physiol 247(2 Pt 2):F282–F290
Cole JA, Forte LR, Krause WJ, Thorne PK (1989) Clonal sublines that are morphologically and functionally distinct from parental OK cells. Am J Physiol 256(4 Pt 2):F672–F679
Sekine T, Cha SH, Hosoyamada M, Kanai Y, Watanabe N, Furuta Y et al (1998) Cloning, functional characterization, and localization of a rat renal Na+ -dicarboxylate transporter. Am J Physiol 275(2 Pt 2):F298–F305
Chen XZ, Shayakul C, Berger UV, Tian W, Hediger MA (1998) Characterization of a rat Na+ -dicarboxylate cotransporter. J Biol Chem 273(33):20972–20981
Chen X, Tsukaguchi H, Chen XZ, Berger UV, Hediger MA (1999) Molecular and functional analysis of SDCT2, a novel rat sodium-dependent dicarboxylate transporter. J Clin Invest 103(8):1159–1168
Kekuda R, Wang H, Huang W, Pajor AM, Leibach FH, Devoe LD et al (1999) Primary structure and functional characteristics of a mammalian sodium-coupled high affinity dicarboxylate transporter. J Biol Chem 274(6):3422–3429
Steffgen J, Tolan D, Beery E, Burckhardt G, Muller GA (1999) Demonstration of a Na(+)-dicarboxylate cotransporter in bovine adrenocortical cells. Pflugers Arch 438(6):860–864
Wang H, Fei YJ, Kekuda R, Yang-Feng TL, Devoe LD, Leibach FH et al (2000) Structure, function, and genomic organization of human Na(+)-dependent high-affinity dicarboxylate transporter. Am J Physiol Cell Physiol 278(5):C1019–C1030
Pajor AM, Gangula R, Yao X (2001) Cloning and functional characterization of a high-affinity Na(+)/dicarboxylate cotransporter from mouse brain. Am J Physiol Cell Physiol 280(5):C1215–C1223
Anzai N, Jutabha P, Kanai Y, Endou H (2005) Integrated physiology of proximal tubular organic anion transport. Curr Opin Nephrol Hypertens 14(5):472–479
Burckhardt BC, Lorenz J, Kobbe C, Burckhardt G (2005) Substrate specificity of the human renal sodium dicarboxylate cotransporter, hNaDC-3, under voltage-clamp conditions. Am J Physiol Renal Physiol 288(4):F792–F799
Clapham DE (2003) TRP channels as cellular sensors. Nature 426(6966):517–524
Ward DT, McLarnon SJ, Riccardi D (2002) Aminoglycosides increase intracellular calcium levels and ERK activity in proximal tubular OK cells expressing the extracellular calcium-sensing receptor. J Am Soc Nephrol 13(6):1481–1489
Bozic M, Valdivielso JM (2012) Calcium signaling in renal tubular cells. Adv Exp Med Biol 740:933–944
Pajor AM (1995) Sequence and functional characterization of a renal sodium/dicarboxylate cotransporter. J Biol Chem 270(11):5779–5785
Acknowledgments
Supported by a grant from the Institutional Award program of the National Center for Research Resources (P20RR017659), NIH DK54952, and a MREP (KSHS) and Merit Review grant (LLH) from the Department of Veterans Affairs.
Conflict of interest
The authors have no conflict of interest to report.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Hering-Smith, K.S., Mao, W., Schiro, F.R. et al. Localization of the calcium-regulated citrate transport process in proximal tubule cells. Urolithiasis 42, 209–219 (2014). https://doi.org/10.1007/s00240-014-0653-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-014-0653-4