Abstract
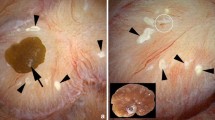
Extensive evidence now supports the role of papillary interstitial deposits—Randall’s plaques—in the formation of stones in the idiopathic, calcium oxalate stone former. These plaques begin as deposits of apatite in the basement membranes of the thin limbs of Henle’s loop, but can grow to become extensive deposits beneath the epithelium covering the papillary surface. Erosion of this covering epithelium allows deposition of calcium oxalate onto this plaque material, and the transition of mineral type and organic material from plaque to stone has been investigated. The fraction of the papilla surface that is covered with Randall’s plaque correlates with stone number in these patients, as well as with urine calcium excretion, and plaque coverage also correlates inversely with urine volume and pH. Two animal models—the NHERF-1 and THP-null mice—have been shown to develop sites of interstitial apatite plaque in the renal papilla. In these animal models, the sites of interstitial plaque in the inner medulla are similar to that found in human idiopathic calcium oxalate stone formers, except that the deposits in the mouse models are not localized solely to the basement membrane of the thin limbs of Henle’s loop, as in humans. This may be due to the different morphology of the human versus mouse papillary region. Both mouse models appear to be important to characterize further in order to determine how well they mimic human kidney stone disease.













Similar content being viewed by others
References
Coe FL, Evan AP, Worcester EM, Lingeman JE (2010) Three pathways for human kidney stone formation. Urol Res 38(3):147–160
Randall A (1937) The origin and growth of renal calculi. Ann Surg 105:1009–1027
Randall A (1940) The etiology of primary renal calculus. Int Abst Surg 71:209–240
Burry AF, Axelsen RA, Trolove P, Sallis JD (1976) Calcification in the renal medulla: a classification based on a prospective study of 2261 necropsies. Hum Pathol 7:435–449
Haggit RC, Pitcock JA (1971) Renal medullary calcifications: a light and electron microscopic study. J Urol 106:342–347
Vermooten V (1942) The origin and development in the renal papilla of Randall’s calcium plaques. J Urol 48:27–31
Anderson LMJR (1946) Origin, frequency and significance of microscopic calculi in kidney. Surg Gynecol Obstetr 82:275–282
Khan SR, Finlayson B, Hackett R (1984) Renal papillary changes in patient with calcium oxalate lithiasis. Urology 23:194–199
Cooke SAR (1970) The site of calcification in the human renal papilla. Br J Surg 57:890–896
Weller RO, Nester B, Cooke SAR (1971) Calcification in the human papilla: an electron-microscope study. J Pathol 107:211–216
Stoller ML, Shami GS, McCormick VD, Kerschmann RL (1996) High resolution radiography of cadaveric kidneys: unraveling the mystery of Randall’s plaque formation. J Urol 156:1263–1266
Low RK, Stoller ML (1997) Endoscopic mapping of renal papillae for Randall’s plaque in patients with urinary stone disease. J Urol 158:2062–2064
Low RK, Stoller ML, Schreiber CK (2000) Metabolic and urinary risk factors associated with Randall’s papillary plaque. J Endourol 14:507–510
Coe FL, Parks JH, Asplin JR (1992) The pathogenesis and treatment of kidney stones. N Engl J Med 327:1141–1152
Evan AP, Lingeman JE, Coe FL, Parks JH, Bledsoe SB, Shao Y, Sommer AJ, Paterson RF, Kuo RL, Grynpas M (2003) Randall’s plaque of patients with nephrolithiasis begins in basement membranes of thin loops of Henle. JCI 111:607–616
Evan AP, Coe FL, Gillen D, Lingeman JE, Bledsoe S, Worcester EM (2008) Renal intra-tubular crystals and hyaluronan staining occur in stone formers with bypass surgery but not with idiopathic calcium oxalate stones. Anat Rec 291:325–334
Evan AP, Coe FL, Rittling SR, Bledsoe SM, Shao Y, Lingeman JE, Worcester EM (2005) Apatite plaque particles in inner medulla of kidneys of calcium oxalate stone formers: osteopontin localizaton. Kidney Intl 68:145–154
Evan AP, Bledsoe S, Worcester EM, Coe FL, Lingeman JE, Bergsland KJ (2007) Renal inter-α-trypsin inhibitor heavy chain 3 increases in calcium oxalate stone-forming patients. Kidney Intl 72:1503–1511
Anderson WAD (1944) Renal calcification in adults. J Urol 44:29–34
Evan AP, Coe FL, Lingeman JE, Shao Y, Anderson JC, Worcester EM (2007) Mechanism of formation of human calcium oxalate renal stones on Randall’s plaque. Anat Rec 290:1315–1323
Kuo RL, Lingeman JE, Evan AP, Paterson RF, Parks JH, Bledsoe SB, Munch LC, Coe FL (2003) Urine calcium and volume predict coverage of renal papilla by Randalls plaque. Kidney Intl 64:2150–2154
Worcester EM, Bergsland K, Evan AP, Parks JH, Coe FL, Willis LR, Clark DL, Gillen D (2008) Evidence for increased postprandial distal nephron calcium delivery in hypercalciuric stone forming patients. AJP 295:F1286–F1294
Kim SC, Tinmouth WW, Coe F, Kuo RL, Paterson RF, Parks J, Evan AP, Lingeman JL (2005) Stone formation is proportional to papillary surface coverage by Randall’s plaque. J Urol 173:117–119
Evan AP, Bledsoe SB (2008) Bone genes in the kidney stone former. In: Evan AP, McAteer JA, Lingeman JE, Williams JC (eds) Renal stone disease. Proceedings of the second international urolithiasis research symposium. American Institute of Physics, Melville, pp 33–43
Weinman EJ, Sreplock D, Shenolikar S (1995) Characterization of a protein co-factor that mediates protein kinase A regulation of the renal brush border membrane Na+-H+ exchanger. J Clin Invest 95:2143–2149
Wade JB, Liu J, Coleman RA, Cunningham R, Steplock DA, Lee-Kwon W, Pallone TL, Shenolikar S, Weinman EJ (2003) Localization and interaction of NHERF isoforms in the renal proximal tubule of the mouse. APJ 285:C1494–C1503
Murer H, Hernando N, Forster I, Biber J (2003) Regulation of Na/Pi transporter in the proximal tubule. Annu Rev Physiol 65:51–542
Voltz JW, Weinman EJ, Shenolikar S (2001) Expanding the role of nherf, a pdz-domain containing protein adapter, to growth regulation. Oncogene 20(44):6309–6314
Shenolikar S, Voltz JW, Minkoff CM, Wade JB, Weinman EJ (2002) Targeted distrution of the mouse NHERF-1 gene promotes internalization of proximal tubule sodium-phosphate cotransporter type IIa and renal phosphate wasting. Proc Natl Acad Sci 99:11470–11475
Chau H, El-Maadawy S, McKee MD, Tenenhouse HS (2003) Renal calcification in mice homozygous for the disrupted type IIa Na/Pi cotransporter gene Npt2. J Bone Miner Res 18:644–657
Kumar V, Lieske JC (2006) Protein regulation of intrarenal crystallization. Curr Opin Nephrol Hypertens 15:374–380
Lau WH, Leong WS, Ismail Z, Gam LH (2008) Qualification and application of an ELISA for the determination of Tamm Horsfall Protein (THP) in human urine and its use for screening of kidney stone disease. Int J Biol Sci 4:215–222
Knorle R, Schnierle P, Koch A, Buchholz NP, Hering F, Seiler H, Ackermann T, Rutishauser G (1994) Tamm-Horsfall glycoprotein: role in inhibition and promotion of renal calcium oxalate stone formation studied with Fourier-transform infrared spectroscopy. Clin Chem 40:1739–1743
Raffi H, Baes JM, Laszik Z, Kumar S (2006) Tamm-Horsfall protein knockout mice do not develop medullary cystic kidney disease. Kidney Intl 69:1914–1915
Liu YL, Mo L, Goldfarb DS, Evan AP, Liang F, Khan SR, Lieske JC, Wu, X-R (2010) Progressive renal papillary calcification and ureteral stone formation in mice deficient for tamm-Horsfall protein. AJP. doi:10.1152/ajprenal.00243.2010
Evan AP, Lingeman JE, Coe FL, Bledsoe SB, Sommer AJ, Williams JC Jr, Krambeck AE, Worcester EM (2009) Intra-tubular deposits, urine and stone composition are divergent in patients with ileostomy. Kidney Int 76:1081–1088
Acknowledgment
This study was funded by NIH P01 DK56788.
Author information
Authors and Affiliations
Corresponding author
Additional information
Proceedings paper from the 3rd International Urolithiasis Research Symposium, Indianapolis, Indiana, USA, December 3-4, 2009.
Rights and permissions
About this article
Cite this article
Evan, A.P., Weinman, E.J., Wu, XR. et al. Comparison of the pathology of interstitial plaque in human ICSF stone patients to NHERF-1 and THP-null mice. Urol Res 38, 439–452 (2010). https://doi.org/10.1007/s00240-010-0330-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00240-010-0330-1