Abstract
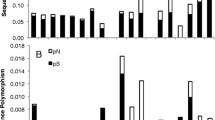
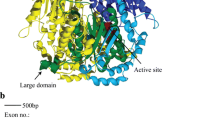
A molecular evolutionary explanation of natural genetic variation requires analysis of specific variants’ evolutionary dynamics. To pursue this for phosphoglucose isomerase (PGI) of Colias butterflies, whose polymorphism is maintained by strong natural selection, we assembled a large data set of wild haplotypes, highly variable at the amino acid and DNA levels. The most common electrophoretic, i.e., charge macrostate, allele class, 3, is conserved in its pattern of charged amino acid residues. The next most common macrostate, 4, has multiple patterns of charge, i.e., microstates, while less common (1, 2, 5, 6) macrostates are very diverse. Macrostate 4 shows significant linkage disequilibrium (LD) among its variants, especially for two groups of five haplotypes each. We find extensive intragenic recombination among all haplotypes except the two high-LD groups of macrostate 4, which display none. Phyletic relations among haplotypes are largely reticulate, again except for the high-LD groups of macrostate 4, which form clades with strong bootstrap support. Charge-changing and linked charge-neutral amino acid variants occur in diverse parts of PGI’s sequence. Homology-based modeling of PGI’s structure shows that these regions are related spatially in ways suggesting functional interaction. The high-LD groups of macrostate 4 display parallel amino acid variation in these regions. This pattern of haplotype clades with high LD among multiple varying sites, emerging from chaotically recombining variation, may be a “signature” of refinement of complex adaptive sequences by recombination and selection. It should be tested further in this study system and others as a possibly general feature of the evolution of living complexity.






Similar content being viewed by others
References
Carter PA, Watt WB (1988) Adaptation at specific loci. V. Metabolically adjacent enzyme loci may have very distinct experiences of selective pressures. Genetics 119:913–924
Dahlhoff EP, Rank NE (2000) Functional and physiological consequences of genetic variation at phosphoglucose isomerase: heat shock protein expression is related to enzyme genotype in a montane beetle. Proc Natl Acad Sci USA 97:10056–10061
de Massy B (2003) Distribution of meiotic recombination sites. Trends Genet 19:514–522
Ellegren H, Sheldon BC (2008) Genetic basis of fitness differences in natural populations. Nature 452:169–175
Eswar N, Webb B, Marti-Renom MA, Madhusudhan MS, Earmian D, Shen M, Pieper U, Sali A (2006) Comparative protein structure modelling using Modeller. Curr Prot Bioinform 5.6:1–30. http://salilab.org/modeller/]
Feder ME, Mitchell-Olds T (2003) Ecological and evolutionary functional genomics. Nature Rev Genet 4:649–655
Feder ME, Watt WB (1992) Functional biology of adaptation. In: Berry RJ, Crawford TJ, Hewitt GM (eds) Genes in ecology. British Ecological Society/Blackwell Scientific, Oxford, pp 365–392
Felsenstein J (2005) PHYLogeny Inference Package, v. 3.63. http://evolution.gs.washington.edu/phylip.html
Filatov DA, Charlesworth D (1999) DNA polymorphism, haplotype structure, and balancing selection in the Leavenworthia PgiC locus. Genetics 153:1423–1434
Ginalski K, Elofsson A, Fischer D, Rychlewski L (2003) 3D-Jury: a simple approach to improve protein structure predictions. Bioinformatics 19:1015–1018. http://meta.bioinfo.pl/
Goldstein A (1964) Biostatistics. Macmillan, New York
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704. http://atgc.lirmm.fr/phyml/]
Hall B (1982) Evolution on a petri dish. Evol Biol 15:85–150
Hall T (2004) BioEdit: biological sequence alignment editor. http://www.mbio.ncsu.edu/BioEdit/bioedit.html
Hanski I, Saccheri I (2006) Molecular-level variation affects population growth in a butterfly metapopulation. PloS Biol 4:719–726
Heyer W-D (2004) Recombination: holliday junction resolution and crossover formation. Curr Biol 14:R56–R58
Hudson RR, Kaplan NL (1985) Statistical properties of the number of recombination events in the history of a sample of DNA sequences. Genetics 111:147–164
Hudson RR, Bailey K, Skarecky D, Kwiatowski J, Ayala FJ (1994) Evidence for positive selection in the superoxide dismutase (Sod) region of Drosophila melanogaster. Genetics 136:1329–1240
Jeffery CJ, Bahnson BJ, Chien W, Ringe D, Petsko GA (2000) Crystal structure of rabbit phosphoglucose isomerase, a glycolytic enzyme that moonlights as neuroleukin, autocrine mobility factor, and differentiation mediator. Biochemistry 39:955–964
Katz LA, Harrison RG (1997) Balancing selection on electrophoretic variation of phosphoglucose isomerase in two species of field cricket: Gryllus veletis and G. pennsylvanicus. Genetics 147:609–621
Kreitman M (1983) Nucleotide polymorphism at the alcohol dehydrogenase locus of Drosophila melanogaster. Nature 304:412–417
Leslie JF, Watt WB (1986) Some evolutionary consequences of the molecular recombination process. Trends Genet 2:288–291
Lewontin RC (2000) The problems of population genetics. In: Singh RS, Krimbas CB (eds) Evolutionary genetics: from molecules to morphology. Cambridge University Press, Cambridge, pp 5–23
Mitchell-Olds T, Schmitt JM (2006) Genetic mechanisms and evolutionary significance of natural variation in Arabidopsis. Nature 441:947–952
Patarnello T, Battaglia B (1992) Glucosephosphate isomerase and fitness: effects of temperature on genotype dependent mortality and enzyme activity in two species of the genus Gammarus (Crustacea, Amphipoda). Evolution 46:1568–1573
Rank NE, Dahlhoff EP (2002) Allele frequency shifts in response to climate change and physiological consequences of allozyme variation in a montane insect. Evolution 56:2278–2289
Rozas J, Sanchez-DelBarrio JC, Messeguer X, Rozas R (2003) DnaSP, DNA polymorphism analyses by the coalescent and other methods. Bioinformatics 19:2496–2497. http://www.ub.edu/dnasp/
Schwede, T, Kopp, J, Guex, N, Peitsch MC (2003) SWISS-MODEL: an automated protein homology-modelling server. Nucleic Acids Res 31:3381–3385. http://swissmodel.expasy.org
Shaw PJ, Muirhead H (1977) Crystallographic structure analysis of glucose 6-phosphate isomerase at 3.5 Ǻ resolution. J Mol Biol 109:475–485
Snyder LRG, Hayes P, Chappell MA (1988) α-Chain hemoglobin polymorphisms are correlated with altitude in the deer mouse, Peromyscus maniculatus. Evolution 42:689–697
Sokal RR, Rohlf FJ (1995) Biometry, 3rd edn. W. H. Freeman, New York
Solomons JTG, Zimmerly EM, Burns S, Krishnamurthy N, Swan MK, Krings S, Muirhead H, Chirgwin J, Davies C (2004) The crystal structure of mouse phosphoglucose isomerase at 1.6 Ǻ resolution and its complex with glucose-6-phosphate reveals the catalytic mechanism of sugar-ring opening. J Mol Biol 342:847–860
Steiner CC, Weber JN, Hoekstra HE (2007) Adaptive variation in beach mice produced by two interacting pigmentation genes. PLoS Biol 5:1880–1889
Storz JF, Sabatino SJ, Hoffmann FG, Gering EJ, Moriyama H, Ferrand N, Monteiro B, Nachman MW (2007) The molecular basis of high-altitude adaptation in deer mice. PloS Genetics 3:448–459
Tajima F (1989) Statistical method for testing the neutral mutation hypothesis by DNA polymorphism. Genetics 123:585–595
Tian D, Araki H, Stahl E, Bergelson J, Kreitman M (2002) Signature of balancing selection in Arabidopsis. Proc Natl Acad Sci USA 99:11525–11530
Van Beneden RJ, Powers DA (1989) Structural and functional differentiation of two clinally distributed glucosephosphate isomerase allelic isozymes from the teleost, Fundulus heteroclitus. Mol Biol Evol 6:155–170
Verrelli BC, Eanes WF (2001a) Clinal variation for amino acid polymorphisms at the Pgm locus in Drosophila melanogaster. Genetics 157:1649–1663
Verrelli BC, Eanes WF (2001b) The functional impact of Pgm amino acid polymorphism on glycogen content in Drosophila melanogaster. Genetics 159:201–210
Watt WB (1972) Intragenic recombination as a source of population genetic variability. Amer Nat 106:737–753
Watt WB (1977) Adaptation at specific loci. I. Natural selection on phosphoglucose isomerase of Colias butterflies: biochemical and population aspects. Genetics 87:177–194
Watt WB (1983) Adaptation at specific loci II. Demographic and biochemical elements in the maintenance of the Colias PGI polymorphism. Genetics 103:691–724
Watt WB (1992) Eggs, enzymes, and evolution—natural genetic variants change insect fecundity. Proc Natl Acad Sci USA 89:10608–10612
Watt WB (2003) Mechanistic studies of butterfly adaptations. In: Boggs CL, Watt WB, Ehrlich PR (eds) Butterflies: ecology and evolution taking flight. University of Chicago Press, Chicago, pp 319–352
Watt WB, Dean AM (2000) Molecular-functional studies of adaptive genetic variation in prokaryotes and eukaryotes. Annu Rev Genet 34:593–622
Watt WB, Donohue K, Carter PA (1996) Adaptation at specific loci. VI. Divergence vs. parallelism of polymorphic allozymes in molecular function and fitness—component effects among Colias species (Lepidoptera, Pieridae). Mol Biol Evol 13:699–709
Watt WB, Wheat CW, Meyer EH, Martin J-F (2003) Adaptation at specific loci. VII. Natural selection, dispersal, and the diversity of molecular-functional variation patterns among butterfly species complexes (Colias: Lepidoptera, Pieridae). Mol Ecol 12:1265–1275
Weinreich DM, Delaney NF, De Pristo MA, Hartl DL (2006) Darwinian evolution can follow only very few mutational paths to fitter proteins. Science 312:111–114
Wheat CW, Watt WB (2008) A mitochondrial-DNA-based phylogeny for some evolutionary-genetic model species of Colias butterflies (Lepidoptera, Pieridae). Mol Phylogenet Evol 47:893–902
Wheat CW, Watt WB, Boutwell CL (2005) A reconnaissance of population genetic variation in arctic and subarctic sulfur butterflies (Colias: Lepidoptera, Pieridae). Can J Zool 83:1614–1623
Wheat CW, Watt WB, Pollock DD, Schulte PM (2006) From DNA to fitness differences: sequences and structures of adaptive variants of Colias phosphoglucose isomerase (PGI). Mol Biol Evol 23:499–512
Zamer W, Hoffmann RJ (1989) Allozymes of glucose-6-phosphate isomerase differentially modulate pentose-shunt metabolism in the sea anemone Metridium senile. Proc Natl Acad Sci USA 86:2737–2741
Acknowledgments
We thank Carol Boggs, Chris Davies, Jason Hill, Richard Hudson, Martin Kreitman, Magnus Nordborg, Dmitri Petrov, and Ben Webb for comments on the paper and/or other helpful discussions; Chris Wheat for the use of C. meadii sequence m2-196; Nicole Bui, Mason DePasse, Alejandro Perez, Brian Pfeiffer, and Gurnek Singh for technical assistance; and the U.S. National Science Foundation for grant support (DEB 05-20315 to W.W.). Our findings are our own and represent no official policy of any government institution or corporate entity.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Wang, B., Watt, W.B., Aakre, C. et al. Emergence of Complex Haplotypes from Microevolutionary Variation in Sequence and Structure of Colias Phosphoglucose Isomerase. J Mol Evol 68, 433–447 (2009). https://doi.org/10.1007/s00239-009-9237-2
Received:
Revised:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00239-009-9237-2