Abstract
Purpose
Development of best practices for dealing with incidental findings on neuroimaging requires insight in their frequency and clinical relevance.
Methods
Here, we delineate prevalence estimates with 95% confidence intervals and clinical management of incidental findings, based on the first 3589 participants of the population-based Rhineland Study (age range 30–95 years) who underwent 3 Tesla structural neuroimaging (3D, 0.8 mm3 isotropic resolution). Two trained raters independently assessed all scans for abnormalities, with confirmation and adjudication where needed by neuroradiologists. Participants were referred for diagnostic work-up depending on the potential benefit.
Results
Of 3589 participants (mean age 55 ± 14 years, 2072 women), 867 had at least one possible incidental finding (24.2%). Most common were pituitary abnormalities (12.3%), arachnoid cysts (4.1%), developmental venous anomalies (2.5%), non-acute infarcts (1.8%), cavernomas (1.0%), and meningiomas (0.7%). Forty-six participants were informed about their findings, which was hitherto unknown in 40 of them (1.1%). Of these, in 19 participants (48%), a wait-and-see policy was applied and nine (23%) received treatment, while lesions in the remainder were benign, could not be confirmed, or the participant refused to inform us about their clinical diagnosis.
Conclusion
Nearly one-quarter of participants had an incidental finding, but only 5% of those required referral, that mostly remained without direct clinical consequences.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Magnetic resonance imaging (MRI) has been widely used in both research and clinical practice over the past decades. As a consequence, people had to develop best practices for dealing with incidental findings. An incidental finding is a previously unknown abnormality of potential clinical relevance that is unexpectedly discovered and unrelated to the specific research purposes of a study itself [1].
The prevalence of incidental findings on neuroimaging varies across studies depending on the age distribution of participants and the imaging modalities used [2]. So far, population-based studies have reported incidental findings mostly in older people and using 1.5 Tesla neuroimaging [3,4,5,6,7] with only a few studies using at least one 3D imaging sequence [5,6,7]. To the best of our knowledge, the Study of Health in Pomerania study is the only population-based study that reported on incidental findings on MRI covering a broad age range by including participants aged between 21 and 88 years; however, their imaging protocol was limited to 2D MR images [8].
Based on the large, single-center population-based Rhineland Study, we here report on the prevalence of incidental findings detected on brain neuroimaging using 0.8 mm3 isotropic 3D imaging sequences across the adult life span, and provide information about clinical management of incidental findings that were reported back to the participant.
Methods
Study population
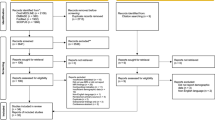
This study is based on all participants who underwent structural brain MRI out of the first 5000 consecutive participants of the Rhineland Study (n = 3589, shown in Fig. 1). The Rhineland Study is an ongoing, prospective, single-center, community-based cohort study. All inhabitants aged 30–100 years of two geographically defined areas in Bonn, Germany, are invited to participate in the study. The sole exclusion criterion is insufficient command of the German language to provide informed consent.
Magnetic resonance imaging data acquisition
MRI data was acquired on 3 Tesla MRI scanners (Siemens Prisma Magnetom, Erlangen, Germany) equipped with an 80 mT/m gradient system and a 64-channel phased-array head-neck coil, including the following in-house developed sequences: a 3D T1-weighted multi-echo magnetization prepared rapid gradient-echo (ME-MPRAGE) sequence (time of acquisition (TA) = 6.5 min, repetition time (TR) = 2560 ms, inversion time (TI) = 1100 ms, flip angle 7°, field of view (FOV) = 256 × 256 mm, 0.8 mm isotropic) [9, 10]; a 3D T2-weighted Turbo-Spin-Echo (TSE) (TA = 4.6 min, TR = 2800 ms, echo time (TE) = 405 ms, FOV = 256 × 256 mm, 0.8 mm isotropic) [11, 12]; and a 3D T2 fluid-attenuated inversion recovery (FLAIR) pulse sequence (TA = 4.5 min, TR = 5000 ms, TE = 393 ms, TI = 1800 ms, FOV = 256 × 256 mm, 1.0 mm isotropic). All sequences employ parallel imaging acceleration with CAIPIRINHA sampling [13] and elliptical sampling [14].
For the initial screening of incidental findings, all images were reconstructed to a resolution of 2.5 mm isotropic to reduce the workload of the reader. When an abnormality was seen, the reader had direct access to the original images for detailed assessment.
Assessment and clinical management of incidental findings
The workflow of the assessment of incidental findings in the Rhineland Study is depicted in Fig. 2. Criteria for what constitutes an incidental finding and which findings should be reported back to the participant were developed by an expert committee based on clinical guidelines, state-of-the-art scientific evidence, and ethical considerations (see Table 1). Possible incidental findings that were explicitly, but not exclusively, checked for included infarcts, hemorrhage, malignant tumors, parenchymal brain lesions, intraventricular lesions, pituitary lesions, brainstem lesions, lesions involving a cranial nerve, meningiomas, arachnoid cysts, aneurysms, arteriovenous malformations, cavernous malformations, developmental venous malformations, developmental abnormalities, and white matter hyperintensities that were presumably not due to cerebral small vessel disease (including multiple sclerosis). The latter was based on the dark appearance of white matter hyperintensities on T1-weighted images as well as the clinical experience of the neuroradiologists. Initial readings with this prespecified protocol were performed with OsiriX MD, an image processing application for DICOM images, by two of three independent raters (VL, cognitive neuroscientist with 6 years of experience (until end of study); RL, radiologist with 7 years of experience (until August 2019); specifically trained medical student with 1 year of experience (from August 2019 onwards)). The initial raters had previous experience in MR image reading in clinical routine or for research purposes. Additionally, before the start of the study, they joined the Clinic for Neuroradiology in Bonn for 2 weeks to get more specific training in the detection of brain abnormalities, and had specific training sessions with neuroradiologists (e.g., to distinguish between normal variations and cystic lesions of the pituitary gland). To train new raters, they developed an initial training set including 110 MRI scans from the Rhineland Study, which included both scans with and without abnormalities. The third rater got trained using this initial training set as well as 150 additional random MRI scans from the Rhineland Study. The training set is still increasing in size as raters continue to include interesting cases.
The initial ratings were done blinded to the medical history of participants, usually within 1 working day by at least one of the raters. Next, both ratings were compared. In case of persistent disagreement, an incidental finding that possibly would require referral, or whenever further clarification was needed, an experienced (neuro-)radiologist also read the images and made a final decision on the classification of the finding (SJE, radiologist with 7 years of experience; EH, neuroradiologist with 23 years of experience). All judgements were solely made on the basis of the MRI scans.
The decision whether or not to refer a participant with an incidental finding to a medical specialist for clinical work-up depended on the potential benefit for the participant, which was defined a priori by the expert committee mentioned above (Table 1). In case of ethically challenging findings, further experts could be consulted. When referral was needed, a study physician informed the participant and, with the consent of the participant, their general practitioner. Note that we only received feedback on the detected brain abnormality from the persons who we approached for referral. Therefore, we cannot exclude that some of the non-referred lesions were already known to the participant, and therefore in sensu stricto not an incidental finding, even though they had not been reported during the interview.
To obtain information on clinical management of referred abnormalities, we asked the participants to send relevant medical letters or to give consent for us to contact their practitioner to review medical records directly. We only considered clinical diagnoses made by medical specialists after clinical neuroimaging.
Assessment of demographic variables
Hypertension was defined as systolic blood pressure ≥ 140 mmHg, diastolic blood pressure ≥ 90 mmHg, or antihypertensive medication use; diabetes as fasting plasma glucose level ≥ 7 mmol/l, HbA1c ≥ 6.5%, or use of antidiabetic medication. History of multiple sclerosis and stroke, smoking status (current/non-smoker), and education (low, ISCED 0–3; middle, ISCED 4–6; high, ISCED 7–8)[15] was self-reported.
Data availability
The data for this manuscript are not publicly available due to data protection regulations. Access to data can be provided to scientists in accordance with the Rhineland Study’s Data Use and Access Policy. Requests for additional information and/or access to the datasets can be send to RS-DUAC@dnze.de.
Statistical analysis
We calculated the prevalence with 95% confidence intervals (CI) for each incidental finding in our study population. For the most frequent incidental findings, we further evaluated whether prevalence differed between sexes and across age using logistic regression. Multiple similar incidental findings within one participant were counted as a single finding (e.g., multiple arachnoid cysts). P-values < 0.05 were considered as statistically significant. All statistical analyses were performed using R version 4.0.2 [16].
Results
Mean age of the study population was 55 ± 14 years, 58% were women (Table 2). Men compared to women were on average more often higher educated (65 vs. 48%, p = 0.001), were more likely to have diabetes (6 vs. 3%, p < 0.001) and hypertension (40 vs. 34%, p < 0.001), and a higher body mass index (26.1 vs. 25.3, p < 0.001). Participants who underwent MRI were on average younger (55 vs. 56 years, p < 0.001), more often higher educated (55 vs. 47%, p < 0.001), were less likely to have diabetes (5 vs. 7%, p < 0.001), or hypertension (37 vs. 42%, p < 0.001), and had a lower body mass index (25.6 vs. 26.7, p < 0.001), compared to those who did not. Also, more men than women (47 vs. 42%, p = 0.005) were excluded from or refused MRI.
In total, 867 of 3589 participants had at least one possible incidental finding (24.2% [95% CI 22.8–25.6%]) (Table 3). This did not differ between women (505 of 2072 with an incidental finding (24.4% [95% CI 22.5–26.3%])) and men (362 of 1517 (23.9% [95% CI 21.7–26.1%])) (p = 0.764). The maximum number of incidental findings for a single person was four; one participant had an arachnoid cyst, a developmental venous anomaly, a cavernoma, and a possibly malignant lesion; another participant had an arachnoid cyst, cystic lesion of the pituitary gland, inflammatory WM lesions, and cystic lesions around the brainstem. Most frequent incidental findings were pituitary abnormalities (12.3% [95% CI 11.3–13.5%]), arachnoid cysts (4.1% [95% CI 3.5–4.8%]), developmental venous anomalies (2.5% [95% CI 2.0–3.0%]), non-acute infarcts (1.8% [95% CI 1.4–2.3%]), cavernomas (1.0% [95% CI 0.7–1.4%]), and meningiomas (0.7% [95% CI 0.5–1.1%], mean size of the largest dimension, 14.9 ± 6.8 mm). Men had more non-acute infarcts, more arachnoid cysts, and more developmental abnormalities than women (2.3 vs. 1.4%, p = 0.040; 5.2 vs. 3.3%, p = 0.006; 0.7 vs. 0.1%, p = 0.015, respectively). Women had slightly more meningiomas, but because of small numbers, the difference was only borderline significant (1.0 vs. 0.4%, p = 0.056). The presence of non-acute infarcts increased with age (prevalence odds ratio (OR) 1.06 [95% CI 1.04–1.08] per year, p < 0.001), as did the frequency of cavernomas (OR 1.03 [95% CI 1.00–1.05] per year, p = 0.044), and of meningiomas (OR 1.05 [95% CI 1.02–1.08] per year, p = 0.002). For other incidental findings, we saw no effect of age on prevalence.
Most of the 433 pituitary anomalies that we found were pituitary cysts (mostly pars intermedia cysts; 95.9% [95% CI 93.7–97.6%]); the remainder were (semi-)solid lesion with or without a mass effect, most likely to be microadenomas. The prevalence of pituitary cysts did not significantly differ between men (10.9% [95% CI 9.4–12.6%]) and women (12.5% [95% CI 11.1–14.0%]) (p = 0.174) and was stable across the adult life span (OR 1.00 [95% CI 0.99–1.01] per year, p = 0.805). The prevalence of other pituitary anomalies did not differ between sexes (women 0.4% [95% CI 0.2–0.8%]; men 0.7% [95% CI 0.3–1.2%]; p = 0.187) but increased with age (OR 1.04 [95% CI 1.01–1.08] per year, p = 0.024).
The raters had initial disagreement in the reading of the MR images in approximately 12% of the cases, where one of the raters had missed an abnormality. Persistent disagreement occurred in less than 1%, where clarification by the neuroradiologist was needed.
Referrals and clinical management
Table 4 shows the subsequent clinical management of the 40 participants who we referred for further diagnostic work-up. They underwent clinical MRI which led to a wait-and-see policy for 19, and treatment for nine participants. In four participants, the findings were confirmed but classified as benign lesions that did not require further therapy or follow-up. Three participants refused to give information on their clinical diagnosis.
The initial finding on basis of the research examination was not confirmed in five of the 40 participants (13% [95% CI 4–27%]). In these five participants, we found signal changes of unclear pathogenesis. In two participants, we found cystic lesions of which one could possibly affect the brainstem and the other might possibly cause a hydrocephalus. In two participants, we observed signal changes around the amygdala and in another one changes in the anterior communicating artery which were surrounded by an artefact. In all those cases, we could not rule out malignant pathology and therefore referred these participants for clinical work-up.
Additionally, we found abnormalities that would have required referral according to our protocol in six participants, but were already known and under treatment, and hence by definition no incidental finding.
We did not find any acute lesions that required immediate medical attention, nor any ethically challenging findings for which we would have needed to consult further experts.
Discussion
In this population-based neuroimaging study among 3589 participants of the Rhineland Study, we found incidental brain abnormalities on MRI in approximately one-quarter of all participants, with pituitary cysts being most common. Based on a prespecified protocol, we had to refer 1.1% of all participants for further diagnostic work-up, mostly because of meningiomas, lesions affecting the brainstem, aneurysms, and mass. Subsequent clinical management in the majority of these participants was confined to a wait-and-see policy. One-fifth of those who were referred, or 0.3% of the total sample that had brain imaging, underwent treatment which was successful and without complications.
Consistent with previous reports [5, 17,18,19], we found that men had more arachnoid cysts and non-acute infarcts than women, whereas women had slightly more meningiomas, and that the prevalence of non-acute infarcts and meningiomas increased with age. Contrary to a previous population-based study in older adults, we observed an effect of age on the prevalence of cavernomas [20]. However, the other study only assessed axial T2*-weighted (slice thickness 3.3 mm) or standard T2-weighted images, and their reported prevalence of 0.4% may have been too low to detect age-dependencies.
The prevalence of incidental findings is highly dependent on imaging modalities, with more abnormalities being detected when using at least one high spatial resolution 3D sequence [2, 21]. We found pituitary cysts in 11.8% and arachnoid cysts in 4.1% of our population, which is indeed much higher compared to previous studies reporting frequencies in the range of 0.8–1.8% and 1.4–3.6%, respectively [4,5,6,7,8]. This is likely due to the high spatial resolution of our 3D T2-weighted sequence. The prevalence of aneurysms (0.2%) in our cohort is low compared to previous large cohort studies [4,5,6], which, however, used different imaging modalities, including 2D T2-weighted images or time-of-flight angiography. Our imaging protocol was indeed not optimized to detect aneurysms. Particularly, our highly accelerated 3D T2-weighted sequence is prone to pulsation artefacts interfering with regular intraluminal flow void, making it less suitable for detecting aneurysms.
Discrepancies in prevalence estimates of incidental findings might also be due to classification of what constitutes an incidental finding. For example, we did not include lacunar stroke in non-acute infarcts nor did we track any normal variants (e.g., megacisterna magna).
While the raters initially disagreed in approximately 12% of the cases, this persisted in only less than 1% after an initial consensus meeting. This highlights that it is common for non-radiologist raters to miss small abnormalities on brain MRI scans, and the importance of the four-eye-principle in the reading of MR images in large cohort studies. As the clinical neuroradiologists were not involved in the initial ratings, we could not compare their performance with that of the study raters.
Following our protocol to only refer participants for clinical work-up if this would be of clear potential benefit for the person involved, we only referred 1.1% of the participants suggesting that most abnormalities have no direct clinical consequence. This is in line with reports on potentially clinically relevant incidental findings in adults from a recent meta-analysis and another German cohort [8, 21]. Five of the findings we referred were not confirmed on clinical MRI. Here, we could not rule out possible malignant pathogenesis based on our MRI sequences which were developed for the specific research purposes of the Rhineland Study and not used in clinical settings before. Our prospective follow-up may show whether the lesions we found were indeed false-positive ratings, or that our sequences are more sensitive to subtle changes that are not detectable yet on clinical MRI scans.
Participants included in this study were relatively healthy, as we had to exclude older and sicker people due to MRI contraindications. Additionally, roughly 14% of eligible participants refused MRI. This may have resulted in a further selection bias and the prevalence estimates should be considered a conservative estimate of the true population prevalence of incidental abnormalities.
Major strengths of this study were that it involves a large number of participants drawn from a population-based cohort with a wide age range. We performed state-of-the-art brain MRI including 3D T2-weighted, 3D T1-weighted, and 3D FLAIR sequences, contacted those affected with abnormalities, and followed up concerning their clinical course. All images were reviewed within one working day by at least one experienced reader.
When interpreting the results of this study, some issues should be considered. Our rating of incidental findings was limited to T1-weighted, T2-weighted, and FLAIR images and we did not apply any contrast agents. This may have restricted the number of incidental findings detected and may explain some differences in our prevalence estimates compared to previous studies. Furthermore, we do not have longitudinal data on the natural course of incidental findings yet. The prospective nature of the Rhineland Study, however, will allow us to obtain these in the future.
Conclusions
In conclusion, incidental findings on neuroimaging across the adult life span are common, yet direct clinical consequences are rare. With the number of research studies using high spatial resolution 3D MR neuroimaging sequences rapidly increasing, it is important to have prespecified guidelines on assessing and managing incidental findings. Our procedure and findings can help guiding in the further development of protocols for new research studies.
Data availability
The data for this manuscript are not publicly available due to data protection regulations. Access to data can be provided to scientists in accordance with the Rhineland Study’s Data Use and Access Policy. Requests for additional information and/or access to the datasets can be send to RS-DUAC@dzne.de.
Change history
13 January 2022
A Correction to this paper has been published: https://doi.org/10.1007/s00234-021-02880-y
References
Wolf SM, Lawrenz FP, Nelson CA, Kahn JP, Cho MK, Clayton EW, Fletcher JG, Georgieff MK, Hammerschmidt D, Hudson K, Illes J, Kapur V, Keane MA, Koenig BA, Leroy BS, McFarland EG, Paradise J, Parker LS, Terry SF, Van Ness B, Wilfond BS (2008) Managing incidental findings in human subjects research: analysis and recommendations. J Law Med Ethics 36(2):219–48, 11. https://doi.org/10.1111/j.1748-720X.2008.00266.x
Orme NM, Fletcher JG, Siddiki HA, Harmsen WS, O’Byrne MM, Port JD, Tremaine WJ, Pitot HC, McFarland EG, Robinson ME, Koenig BA, King BF, Wolf SM (2010) Incidental findings in imaging research: evaluating incidence, benefit, and burden. Arch Intern Med 170(17):1525–1532. https://doi.org/10.1001/archinternmed.2010.317
Vernooij MW, Ikram MA, Tanghe HL, Vincent AJ, Hofman A, Krestin GP, Niessen WJ, Breteler MM, van der Lugt A (2007) Incidental findings on brain MRI in the general population. N Engl J Med 357(18):1821–1828. https://doi.org/10.1056/NEJMoa070972
Sandeman EM, Hernandez Mdel C, Morris Z, Bastin ME, Murray C, Gow AJ, Corley J, Henderson R, Deary IJ, Starr JM, Wardlaw JM (2013) Incidental findings on brain MR imaging in older community-dwelling subjects are common but serious medical consequences are rare: a cohort study. PLoS ONE 8(8):e71467. https://doi.org/10.1371/journal.pone.0071467
Bos D, Poels MM, Adams HH, Akoudad S, Cremers LG, Zonneveld HI, Hoogendam YY, Verhaaren BF, Verlinden VJ, Verbruggen JG, Peymani A, Hofman A, Krestin GP, Vincent AJ, Feelders RA, Koudstaal PJ, van der Lugt A, Ikram MA, Vernooij MW (2016) Prevalence, clinical management, and natural course of incidental findings on brain MR images: the population-based Rotterdam Scan Study. Radiology 281(2):507–515. https://doi.org/10.1148/radiol.2016160218
Haberg AK, Hammer TA, Kvistad KA, Rydland J, Muller TB, Eikenes L, Garseth M, Stovner LJ (2016) Incidental intracranial findings and their clinical impact; the HUNT MRI study in a general population of 1006 participants between 50–66 years. PLoS ONE 11(3):e0151080. https://doi.org/10.1371/journal.pone.0151080
Boutet C, Vassal F, Celle S, Schneider FC, Barthelemy JC, Laurent B, Barral FG, Roche F (2017) Incidental findings on brain magnetic resonance imaging in the elderly:the PROOF study. Brain Imaging Behav 11(1):293–299. https://doi.org/10.1007/s11682-016-9519-4
Hegenscheid K, Seipel R, Schmidt CO, Volzke H, Kuhn JP, Biffar R, Kroemer HK, Hosten N, Puls R (2013) Potentially relevant incidental findings on research whole-body MRI in the general adult population: frequencies and management. Eur Radiol 23(3):816–826. https://doi.org/10.1007/s00330-012-2636-6
van der Kouwe AJW, Benner T, Salat DH, Fischl B (2008) Brain morphometry with multiecho MPRAGE. Neuroimage 40(2):559–569. https://doi.org/10.1016/j.neuroimage.2007.12.025
Brenner D, Stirnberg R, Pracht ED, Stocker T (2014) Two-dimensional accelerated MP-RAGE imaging with flexible linear reordering. MAGMA 27(5):455–462. https://doi.org/10.1007/s10334-014-0430-y
Busse RF, Brau AC, Vu A, Michelich CR, Bayram E, Kijowski R, Reeder SB, Rowley HA (2008) Effects of refocusing flip angle modulation and view ordering in 3D fast spin echo. Magn Reson Med 60(3):640–649. https://doi.org/10.1002/mrm.21680
Mugler JP 3rd (2014) Optimized three-dimensional fast-spin-echo MRI. J Magnet Resonan Imag : JMRI 39(4):745–767. https://doi.org/10.1002/jmri.24542
Breuer FA, Blaimer M, Mueller MF, Seiberlich N, Heidemann RM, Griswold MA, Jakob PM (2006) Controlled aliasing in volumetric parallel imaging (2D CAIPIRINHA). Magn Reson Med 55(3):549–556. https://doi.org/10.1002/mrm.20787
Bernstein MA, Fain SB, Riederer SJ (2001) Effect of windowing and zero-filled reconstruction of MRI data on spatial resolution and acquisition strategy. J Magnet Resonan Imag : JMRI 14(3):270–280. https://doi.org/10.1002/jmri.1183
UNESCO (2012) Institute for Statistics: International Standard Classification of Education ISCED 2011. Montréal
R Core Team (2020) R: a language and environment for statistical computing. In: R Foundation for Statistical Computing, editor. Vienna, Austria
Reeves MJ, Bushnell CD, Howard G, Gargano JW, Duncan PW, Lynch G, Khatiwoda A, Lisabeth L (2008) Sex differences in stroke: epidemiology, clinical presentation, medical care, and outcomes. Lancet Neurol 7(10):915–926. https://doi.org/10.1016/S1474-4422(08)70193-5
Wiemels J, Wrensch M, Claus EB (2010) Epidemiology and etiology of meningioma. J Neurooncol 99(3):307–314. https://doi.org/10.1007/s11060-010-0386-3
Oya S, Kim SH, Sade B, Lee JH (2011) The natural history of intracranial meningiomas. J Neurosurg 114(5):1250–1256. https://doi.org/10.3171/2010.12.JNS101623
Flemming KD, Graff-Radford J, Aakre J, Kantarci K, Lanzino G, Brown RD Jr, Mielke MM, Roberts RO, Kremers W, Knopman DS, Petersen RC, Jack CR Jr (2017) Population-based prevalence of cerebral cavernous malformations in older adults: Mayo Clinic Study of Aging. JAMA Neurol 74(7):801–805. https://doi.org/10.1001/jamaneurol.2017.0439
Gibson LM, Paul L, Chappell FM, Macleod M, Whiteley WN, Al-Shahi Salman R, Wardlaw JM, Sudlow CLM (2018) Potentially serious incidental findings on brain and body magnetic resonance imaging of apparently asymptomatic adults: systematic review and meta-analysis. BMJ 363:k4577. https://doi.org/10.1136/bmj.k4577
Acknowledgements
We thank Prof Dr Aad van der Lugt and Prof Dr Fabian Bamberg for insightful discussions and advice in the development of our protocol for dealing with incidental findings. Additionally, we thank Pauline Bongard for her contribution to the incidental finding grading.
Funding
Open Access funding enabled and organized by Projekt DEAL. Study funding: The Rhineland Study at the DZNE is predominantly funded by the Federal Ministry of Education and Research (BMBF) and the Ministry of Culture and Science of the German State of North Rhine-Westphalia.
Author information
Authors and Affiliations
Contributions
Author contributions included conception and study design (VL, RL, EH, TS, and MMBB), data collection or acquisition (VL, RL, SJE, and EH), statistical analysis (VL), and interpretation of results, drafting the manuscript work or revising it critically for important intellectual content, approval of final version to be published and agreement to be accountable for the integrity and accuracy of all aspects of the work (all authors).
Corresponding author
Ethics declarations
Compliance with ethical standards
Approval to undertake the study was obtained from the ethics committee of the University of Bonn, Medical Faculty. The study is carried out in accordance with the recommendations of the International Council for Harmonization Good Clinical Practice standards. We obtain written informed consent from all participants in accordance with the Declaration of Helsinki.
Consent to participate
All participants signed informed consent for their data to be used for research purposes.
Consent for publication
All participants signed informed consent for their data to be used for research purposes.
Conflict of interest
The authors declare no competing interests.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
The original online version of this article was revised: The original publication “Incidental findings on 3 T neuroimaging: cross-sectional observations from the population-based Rhineland Study”, included the following errors, which have now been corrected:
1) Figure 1 included a typo in the final sample size. Figure 1 has been updated.
2) Table 1: Subheaders were not separated: “Incidental findings that need to be referred for diagnostic work-up’ and 'Incidental findings that do not need diagnostic work-up and are not communicated to the participant”. Table subheaders have been updated. “Malignant tumors, inclusive glioma” has been corrected to “Malignant tumors, including glioma”. The “†” notation after “Acute findings” and “‡” after “Vascular disease” have been deleted. Table layout has been updated to indent some incidental findings.
3) Table 3 footnotes have been updated so the order of the signs are correctly presented.
Rights and permissions
Open Access This article is licensed under a Creative Commons Attribution 4.0 International License, which permits use, sharing, adaptation, distribution and reproduction in any medium or format, as long as you give appropriate credit to the original author(s) and the source, provide a link to the Creative Commons licence, and indicate if changes were made. The images or other third party material in this article are included in the article's Creative Commons licence, unless indicated otherwise in a credit line to the material. If material is not included in the article's Creative Commons licence and your intended use is not permitted by statutory regulation or exceeds the permitted use, you will need to obtain permission directly from the copyright holder. To view a copy of this licence, visit http://creativecommons.org/licenses/by/4.0/.
About this article
Cite this article
Lohner, V., Lu, R., Enkirch, S.J. et al. Incidental findings on 3 T neuroimaging: cross-sectional observations from the population-based Rhineland Study. Neuroradiology 64, 503–512 (2022). https://doi.org/10.1007/s00234-021-02852-2
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-021-02852-2