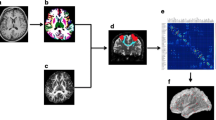
Abstract
Purpose
Burning mouth syndrome (BMS) is a chronic intraoral pain syndrome. Previous studies have attempted to determine the brain connectivity features in BMS using functional and structural magnetic resonance imaging. However, no study has investigated the structural connectivity using multi-shell, multi-tissue-constrained spherical deconvolution (MSMT-CSD), anatomically constrained tractography (ACT), and spherical deconvolution informed filtering of tractograms (SIFT). Therefore, this study aimed to assess the differences in brain structural connectivity of patients with BMS and healthy controls using probabilistic tractography with these methods, and graph analysis.
Methods
Fourteen patients with BMS and 11 age- and sex-matched healthy volunteers underwent 3-T magnetic resonance imaging. MSMT-CSD-based probabilistic structural connectivity was computed using the second-order integration over fiber orientation distributions algorithm based on nodes set in 84 anatomical cortical regions with ACT and SIFT. A t-test was performed for comparisons between the BMS and healthy control brain networks.
Results
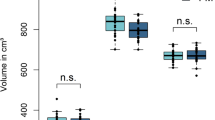
The betweenness centrality was significantly higher in the left insula, right amygdala, and right lateral orbitofrontal cortex and significantly lower in the right inferotemporal cortex in the BMS group than that in healthy controls. However, no significant difference was found in the clustering coefficient, node degree, and small-worldness between the two groups.
Conclusion
Graph analysis of brain probabilistic structural connectivity, based on diffusion imaging using an MSMT-CSD model with ACT and SIFT, revealed alterations in the regions comprising the pain matrix and medial pain ascending pathway. These results highlight the emotional-affective profile of BMS, which is a type of chronic pain syndrome.


Similar content being viewed by others
Data availability
Data cannot be shared publicly because of hospital regulations. Data are available from the University of Tokyo Hospital Institutional Data Access for researchers who meet the criteria for access to confidential data. Please contact the Graduate School of Medicine and Faculty of Medicine, The University of Tokyo Research Ethics Committee (ethics@m.u-tokyo.ac.jp) if you ask for our original data.
Abbreviations
- 5TT:
-
Five-tissue-type
- ACT:
-
Anatomically constrained tractography
- BC:
-
Betweenness centrality
- BMS:
-
Burning mouth syndrome
- CSD:
-
Constrained spherical deconvolution
- EPI:
-
Echo planar imaging
- iFOD2:
-
A second-order integration over fiber orientation distributions
- MRI:
-
Magnetic resonance imaging
- MSMT:
-
Multi-tissue-constrained spherical deconvolution
- PTSD:
-
Post-traumatic stress disorder
- ROI:
-
Region of interest
- SIFT:
-
Spherical deconvolution informed filtering of tractograms
- TE:
-
Echo time
- TR:
-
Repetition time
References
Khan SA, Keaser ML, Meiller TF, Seminowicz DA (2014) Altered structure and function in the hippocampus and medial prefrontal cortex in patients with burning mouth syndrome. Pain 155:1472–1480. https://doi.org/10.1016/j.pain.2014.04.022
Grushka M, Epstein JB, Gorsky M (2002) Burning mouth syndrome. Am Fam Physician 65:615–620
Tan Y, Wu X, Chen J, Kong L, Qian Z (2019) Structural and functional connectivity between the amygdala and orbital frontal cortex in burning mouth syndrome: an fMRI study. Front Psychol 10:1700. https://doi.org/10.3389/fpsyg.2019.01700
Wada A, Shizukuishi T, Kikuta J, Yamada H, Watanabe Y, Imamura Y et al (2017) Altered structural connectivity of pain-related brain network in burning mouth syndrome-investigation by graph analysis of probabilistic tractography. Neuroradiology 59:525–532. https://doi.org/10.1007/s00234-017-1830-2
Behrens TEJ, Berg HJ, Jbabdi S, Rushworth MF, Woolrich MW (2007) Probabilistic diffusion tractography with multiple fibre orientations: what can we gain? Neuroimage 34:144–155. https://doi.org/10.1016/j.neuroimage.2006.09.018
Jeurissen B, Leemans A, Tournier JD, Jones DJ, Sikbers J (2013) Investigating the prevalence of complex fiber configurations in white matter tissue with diffusion magnetic resonance imaging. Hum Brain Mapp 34:2747–2766. https://doi.org/10.1002/hbm.22099
Tuch DS (2004) Q-Ball imaging. Magn Reson Med 52:1358–1372. https://doi.org/10.1002/mrm.20279
Tournier JD, Calamante F, Gadian DG, Connelly A (2004) Direct estimation of the fiber orientation density function from diffusion-weighted MRI data using spherical deconvolution. Neuroimage 23:1176–1185. https://doi.org/10.1016/j.neuroimage.2004.07.037
Tournier JD, Calamante F, Connelly A (2007) Robust determination of the fibre orientation distribution in diffusion MRI: non-negativity constrained super-resolved spherical deconvolution. Neuroimage 35:1459–1472. https://doi.org/10.1016/j.neuroimage.2007.02.016
Wedeen VJ, Hagmann P, Tseng WY, Reese TG, Weisskoff RM (2005) Mapping complex tissue architecture with diffusion spectrum magnetic resonance imaging. Magn Reson Med 54:1377–1386. https://doi.org/10.1002/mrm.20642
Yeh CH, Cho KH, Lin HC, Wang JJ, Lin CP (2008) Reduced encoding diffusion spectrum imaging implemented with a bi-Gaussian model. IEEE Trans Med Imaging 27:1415–1424. https://doi.org/10.1109/TMI.2008.922189
Canales-Rodríguez EJ, Melie-García L, Iturria-Medina Y (2009) Mathematical description of q-space in spherical coordinates: exact q-ball imaging. Magn Reson Med 61:1350–1367. https://doi.org/10.1002/mrm.21917
Wilkins B, Lee N, Gajawelli N, Law M, Leporé N (2015) Fiber estimation and tractography in diffusion MRI: development of simulated brain images and comparison of multi-fiber analysis methods at clinical b-values. Neuroimage 109:341–356. https://doi.org/10.1016/j.neuroimage.2014.12.060
Tur C, Grussu F, Prados F, Charalambous T, Collorone S, Kanber B et al (2020) A multi-shell multi-tissue diffusion study of brain connectivity in early multiple sclerosis. Mult Scler 26(7):774–785. https://doi.org/10.1177/1352458519845105
Calamante F, Jeurissen B, Smith RE, Tournier JD, Connelly A (2018) The role of whole-brain diffusion MRI as a tool for studying human in vivo cortical segregation based on a measure of neurite density. Magn Reson Med 79(5):2738–2744. https://doi.org/10.1002/mrm.26917
Jeurissen B, Tournier JD, Dhollander T, Connelly A, Sijbers J (2014) Multi-tissue constrained spherical deconvolution for improved analysis of multi-shell diffusion MRI data. Neuroimage 103:411–426. https://doi.org/10.1016/j.neuroimage.2014.07.061
Kamagata K, Zalesky A, Hatano T, Di Biase MA, El Samad O, Saiki S et al (2017) Connectome analysis with diffusion MRI in idiopathic Parkinson’s disease: evaluation using multi-shell, multi-tissue, constrained spherical deconvolution. Neuroimage Clin 17:518–529. https://doi.org/10.1016/j.nicl.2017.11.007
Smith RE, Tournier J-D, Calamante F, Connelly A (2013) SIFT: Spherical-deconvolution informed filtering of tractograms. Neuroimage 67:298–312. https://doi.org/10.1016/j.neuroimage.2012.11.049
Sinke MRT, Otte WM, Christiaens D, Schmitt O, Leemans A, van der Toom A et al (2018) Diffusion MRI-based cortical connectome reconstruction: dependency on tractography procedures and neuroanatomical characteristics. Brain Struct Funct 223:2269–2285. https://doi.org/10.1007/s00429-018-1628-y
Smith RE, Tournier JD, Calamante F, Connelly A (2012) Anatomically-constrained tractography: improved diffusion MRI streamlines tractography through effective use of anatomical information. Neuroimage 62:1924–1938. https://doi.org/10.1016/j.neuroimage.2012.06.005
Horbruegger M, Loewe K, Kaufmann J, Wagner M, Schippling S, Pawlitzki M et al (2019) Anatomically constrained tractography facilitates biologically plausible fiber reconstruction of the optic radiation in multiple sclerosis. Neuroimage Clin 22:101740. https://doi.org/10.1016/j.nicl.2019.101740
Smith RE, Tournier JD, Calamante F, Connelly A (2015) The effects of SIFT on the reproducibility and biological accuracy of the structural connectome. Neuroimage 104:253–265. https://doi.org/10.1016/j.neuroimage.2014.10.004
Headache Classification Committee of the International Headache Society (IHS) (2013) The International classification of headache disorders, 3rd edition (beta version). Cephalalgia 33:629–808. https://doi.org/10.1177/0333102413485658
Mugler JP III, Brookeman JR (1990) Three-dimensional magnetization-prepared rapid gradient-echo imaging (3D MP RAGE). Magn Reson Med 15:152–157. https://doi.org/10.1002/mrm.1910150117
Jenkinson M, Beckmann CF, Behrens TE, Woolrich MW, Smith SM (2012) FSL Neuroimage 62:782–790. https://doi.org/10.1016/j.neuroimage.2011.09.015
Tournier JD, Smith R, Raffelt D, Tabbara R, Dhollander T, Pietsch M et al (2019) MRtrix3: a fast, flexible and open software framework for medical image processing and visualisation. Neuroimage 202:116137. https://doi.org/10.1016/j.neuroimage.2019.116137
Veraart J, Novikov DS, Christiaens D, Ades-Aron B, Sijbers J, Fieremans E (2016) Denoising of diffusion MRI using random matrix theory. Neuroimage 142:394–406. https://doi.org/10.1016/j.neuroimage.2016.08.016
Kellner E, Dhital B, Kiselev VG, Reisert M (2016) Gibbs-ringing artifact removal based on local subvoxel-shifts. Magn Reson Med 76:1574–1581. https://doi.org/10.1002/mrm.26054
Andersson JLR, Sotiropoulos SN (2016) An integrated approach to correction for off-resonance effects and subject movement in diffusion MR imaging. Neuroimage 125:1063–1078. https://doi.org/10.1016/j.neuroimage.2015.10.019
Tustison NJ, Avants BB, Cook PA, Zheng Y, Egan A, Yushkevich PA et al (2010) N4ITK: improved N3 bias correction. IEEE Trans Med Imaging 29:1310–1320. https://doi.org/10.1109/TMI.2010.2046908
Dhollander T, Raffelt D, Connelly A (2016) Unsupervised 3-tissue response function estimation from single-shell or multi-shell diffusion MR data without a co-registered T1 image. In: ISMRM Workshop on Breaking the Barriers of Diffusion MRI: 5
Dhollander T, Mito R, Raffelt D, Connelly A (2019) Improved white matter response function estimation for 3-tissue constrained spherical deconvolution. Proc Intl Soc Mag Reson Med: 555
Desikan RS, Ségonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D et al (2006) An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. Neuroimage 31:968–980. https://doi.org/10.1016/j.neuroimage.2006.01.021
Roine T, Jeurissen B, Perrone D, Aelterman J, Philips W, Sijbers J et al (2019) Reproducibility and intercorrelation of graph theoretical measures in structural brain connectivity networks. Med Image Anal 52:56–67. https://doi.org/10.1016/j.media.2018.10.009
Rubinov M, Sporns O (2010) Complex network measures of brain connectivity: uses and interpretations. Neuroimage 52:1059–1069. https://doi.org/10.1016/j.neuroimage.2009.10.003
Rubinov M, Sporns O (2011) Weight-conserving characterization of complex functional brain networks. Neuroimage 56:2068–2079. https://doi.org/10.1016/j.neuroimage.2011.03.069
Brandes U (2001) A faster algorithm for betweenness centrality. J Math Sociol 25:163–177. https://doi.org/10.1080/0022250X.2001.9990249
Onnela JP, Saramäki J, Kertész J, Kaski K (2005) Intensity and coherence of motifs in weighted complex networks. Phys Rev E Stat Nonlin Soft Matter Phys 71:065103. https://doi.org/10.1103/PhysRevE.71.065103
Watts DJ, Strogatz SH (1998) Collective dynamics of “small-world” networks. Nature 393:440–442. https://doi.org/10.1038/30918
Price DD (2000) Psychological and neural mechanisms of the affective dimension of pain. Science 288:1769–1772. https://doi.org/10.1126/science.288.5472.1769
Apkarian AV, Bushnell MC, Treede RD, Zubieta JK (2005) Human brain mechanisms of pain perception and regulation in health and disease. Eur J Pain 9:463–484. https://doi.org/10.1016/j.ejpain.2004.11.001
Veinante P, Yalcin I, Barrot M (2013) The amygdala between sensation and affect: a role in pain. J Mol Psychiatry 1:9. https://doi.org/10.1186/2049-9256-1-9
Neugebauer V (2015) Amygdala pain mechanisms. Handb Exp Pharmacol 227:261–284. https://doi.org/10.1007/978-3-662-46450-2_13
Fanselow MS, LeDoux JE (1999) Why we think plasticity underlying Pavlovian fear conditioning occurs in the basolateral amygdala. Neuron 23:229–232. https://doi.org/10.1016/s0896-6273(00)80775-8
Shin LM, Wright CI, Cannistraro PA, Wedig MM, McMullin K, Martis B et al (2005) A functional magnetic resonance imaging study of amygdala and medial prefrontal cortex responses to overtly presented fearful faces in posttraumatic stress disorder. Arch Gen Psychiatry 62:273–281. https://doi.org/10.1001/archpsyc.62.3.273
Pitman RK, Rasmusson AM, Koenen KC, Shin LM, Orr SP, Gilbertson MW et al (2012) Biological studies of post-traumatic stress disorder. Nat Rev Neurosci 13:769–787. https://doi.org/10.1038/nrn3339
Rauch SL, Whalen PJ, Shin LM, McInerney SC, Macklin ML, Lasko NB et al (2000) Exaggerated amygdala response to masked facial stimuli in posttraumatic stress disorder: a functional MRI study. Biol Psychiatry 47:769–776. https://doi.org/10.1016/s0006-3223(00)00828-3
Williams LM, Kemp AH, Felmingham K, Barton M, Olivieri G, Peduto A et al (2006) Trauma modulates amygdala and medial prefrontal responses to consciously attended fear. Neuroimage 29:347–357. https://doi.org/10.1016/j.neuroimage.2005.03.047
Shin LM, Kosslyn SM, McNally RJ, Alpert NM, Thompson WL, Rauch SL et al (1997) Visual imagery and perception in posttraumatic stress disorder. A positron emission tomographic investigation. Arch Gen Psychiatry 54:233–241. https://doi.org/10.1001/archpsyc.1997.01830150057010
Rauch SL, van der Kolk BA, Fisler RE, Alpert NM, Orr SP, Savage CR et al (1996) A symptom provocation study of posttraumatic stress disorder using positron emission tomography and script-driven imagery. Arch Gen Psychiatry 53:380–387. https://doi.org/10.1001/archpsyc.1996.01830050014003
Liberzon I, Taylor SF, Amdur R, Jung TD, Chamberlain KR, Minoshima S et al (1999) Brain activation in PTSD in response to trauma-related stimuli. Biol Psychiatry 45:817–826. https://doi.org/10.1016/s0006-3223(98)00246-7
Seeley WW, Menon V, Schatzberg AF, Keller J, Glover GH, Kenna H et al (2007) Dissociable intrinsic connectivity networks for salience processing and executive control. J Neurosci 27:2349–2356. https://doi.org/10.1523/JNEUROSCI.5587-06.2007
Eraly SA, Nievergelt CM, Maihofer AX, Barkauskas DA, Biswas N, Agorastos A et al (2014) Assessment of plasma C-reactive protein as a biomarker of posttraumatic stress disorder risk. JAMA Psychiat 71:423–431. https://doi.org/10.1001/jamapsychiatry.2013.4374
Minassian A, Maihofer AX, Baker DG, Nievergelt CM, Geyer MA, Risbrough VB et al (2015) Association of predeployment heart rate variability with risk of postdeployment posttraumatic stress disorder in active-duty marines. JAMA Psychiat 72:979–986. https://doi.org/10.1001/jamapsychiatry.2015.0922
Feller L, Fourie J, Bouckaert M, Khammissa RAG, Ballyram R, Lemmer J (2017) Burning mouth syndrome: aetiopathogenesis and principles of management. Pain Res Manag 2017:1926269. https://doi.org/10.1155/2017/1926269
Jääskeläinen SK (2018) Is burning mouth syndrome a neuropathic pain condition? Pain 159:610–613. https://doi.org/10.1097/j.pain.0000000000001090
Jääskeläinen SK, Woda A (2017) Burning mouth syndrome. Cephalalgia 37:627–647. https://doi.org/10.1177/0333102417694883
Gonçalves L, Dickenson AH (2012) Asymmetric time-dependent activation of right central amygdala neurons in rats with peripheral neuropathy and pregabalin modulation: central amygdala and pregabalin in rat neuropathy. Eur J Neurosci 36:3204–3213. https://doi.org/10.1111/j.1460-9568.2012.08235.x
Acknowledgements
We appreciate Dr. Yoshiki Imamura (Department of Oral Diagnostic Sciences, Nihon University School of Dentistry, Tokyo, Japan) for his professional advice on burning mouth syndrome. We would like to thank Editage (http://www.editage.com) for editing and reviewing this manuscript for English language.
Funding
This work was supported by JSPS KAKENHI Grant Number 16K10330.
Author information
Authors and Affiliations
Contributions
Conceptualization: Ryo Kurokawa, Kouhei Kamiya, Shiori Amemiya, Osamu Abe. Data curation: Ryo Kurokawa, Shimpei Kato, Fumio Suzuki, Shiori Amemiya, Takahiro Shinozaki, Daiki Takanezawa, Ryutarou Kohashi, Osamu Abe. Methodology: Ryo Kurokawa, Kouhei Kamiya, Shiori Amemiya, Takahiro Shinozaki. Formal analysis and investigation: Ryo Kurokawa, Osamu Abe. Writing – original draft preparation: Ryo Kurokawa, Shohei Inui. Writing – review and editing: Kouhei Kamiya, Shimpei Kato, Fumio Suzuki, Shiori Amemiya. Funding acquisition: Osamu Abe. Resources: Takahiro Shinozaki, Daiki Takanezawa, Ryutarou Kohashi. Supervision: Osamu Abe.
Corresponding author
Ethics declarations
Conflict of interest
We have a grant from Siemens Healthcare K.K.
Ethics approval
This multi-institutional prospective study was approved by the local ethics committees [IRB 11742, 2016–21] of the hospitals and performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments.
Consent to participate
Written informed consent was obtained by all study participants.
Consent for publication
All the authors consent to the publication of the manuscript in Neuroradiology.
Additional information
Publisher's note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Supplementary Information
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Kurokawa, R., Kamiya, K., Inui, S. et al. Structural connectivity changes in the cerebral pain matrix in burning mouth syndrome: a multi-shell, multi-tissue-constrained spherical deconvolution model analysis. Neuroradiology 63, 2005–2012 (2021). https://doi.org/10.1007/s00234-021-02732-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-021-02732-9