Abstract
Purpose
Intraoperative MRI (ioMRI) is a valuable tool aiding paediatric brain tumour resection. There is no published evidence comparing the effectiveness of the final intraoperative MRI and early post-operative (24–72 h) MRI as baseline scans following brain tumour resection. We aimed to evaluate whether the final ioMRI scan could serve as the post-operative baseline scan after paediatric brain tumour resections.
Methods
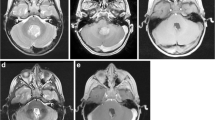
This prospective study compared the final ioMRI scan with the immediate post-operative MRI scan performed 24–72 h post-surgery. We included 20 patients aged 6.6–21 years undergoing brain tumour resection using ioMRI and were suitable for MRI scan without general anaesthesia. The scans were independently evaluated by experienced local and external paediatric neuroradiologists. Identical sequences in the final ioMRI and the 24–72-h MRI were compared to assess the extent of resection, imaging characteristics of residual tumour, the surgical field, extent of surgically induced contrast enhancement, and diffusion abnormalities.
Results
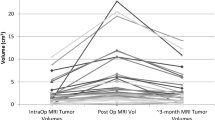
In 20 patients undergoing intraoperative and early post-operative MRI, there was no difference between ioMRI and 24–72-h post-op scans in identifying residual tumour. Surgically induced contrast enhancement was similar in both groups. There were more abnormalities on diffusion imaging and a greater degree of oedema around the surgical cavity on the 24–72-h scan.
Conclusion
The final 3-T ioMRI scan may be used as a baseline post-operative scan provided standard imaging guidelines are followed and is evaluated jointly by the operating neurosurgeon and neuroradiologist. Advantages of final ioMRI as a baseline scan are identified.






Similar content being viewed by others
References
Albert FK, Forsting M, Sartor K, Adams HP, Kunze S (1994) Early postoperative magnetic resonance imaging after resection of malignant glioma: objective evaluation of residual tumor and its influence on regrowth and prognosis. Neurosurgery 34(1):45–60; discussion 60-41. https://doi.org/10.1097/00006123-199401000-00008
Forsyth PA, Petrov E, Mahallati H, Cairncross JG, Brasher P, MacRae ME, Hagen NA, Barnes P, Sevick RJ (1997) Prospective study of postoperative magnetic resonance imaging in patients with malignant gliomas. J Clin Oncol 15(5):2076–2081. https://doi.org/10.1200/JCO.1997.15.5.2076
Oser AB, Moran CJ, Kaufman BA, Park TS (1997) Intracranial tumor in children: MR imaging findings within 24 hours of craniotomy. Radiology 205(3):807–812. https://doi.org/10.1148/radiology.205.3.9393539
Lescher S, Schniewindt S, Jurcoane A, Senft C, Hattingen E (2014) Time window for postoperative reactive enhancement after resection of brain tumors: less than 72 hours. Neurosurg Focus 37(6):E3. https://doi.org/10.3171/2014.9.FOCUS14479
Bette S, Gempt J, Huber T, Boeckh-Behrens T, Ringel F, Meyer B, Zimmer C, Kirschke JS (2016) Patterns and time dependence of unspecific enhancement in postoperative magnetic resonance imaging after glioblastoma resection. World Neurosurg 90:440–447. https://doi.org/10.1016/j.wneu.2016.03.031
Warren KE, Vezina G, Poussaint TY, Warmuth-Metz M, Chamberlain MC, Packer RJ, Brandes AA, Reiss M, Goldman S, Fisher MJ, Pollack IF, Prados MD, Wen PY, Chang SM, Dufour C, Zurakowski D, Kortmann RD, Kieran MW (2018) Response assessment in medulloblastoma and leptomeningeal seeding tumors: recommendations from the Response Assessment in Pediatric Neuro-Oncology committee. Neuro-Oncology 20(1):13–23. https://doi.org/10.1093/neuonc/nox087
Cooney TM, Cohen KJ, Guimaraes CV, Dhall G, Leach J, Massimino M, Erbetta A, Chiapparini L, Malbari F, Kramer K, Pollack IF, Baxter P, Laughlin S, Patay Z, Young Poussaint T, Warren KE (2020) Response assessment in diffuse intrinsic pontine glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol 21(6):e330–e336. https://doi.org/10.1016/S1470-2045(20)30166-2
Fangusaro J, Witt O, Hernaiz Driever P, Bag AK, de Blank P, Kadom N, Kilburn L, Lober RM, Robison NJ, Fisher MJ, Packer RJ, Young Poussaint T, Papusha L, Avula S, Brandes AA, Bouffet E, Bowers D, Artemov A, Chintagumpala M, Zurakowski D, van den Bent M, Bison B, Yeom KW, Taal W, Warren KE (2020) Response assessment in paediatric low-grade glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol 21(6):e305–e316. https://doi.org/10.1016/S1470-2045(20)30064-4
Erker C, Tamrazi B, Poussaint TY, Mueller S, Mata-Mbemba D, Franceschi E, Brandes AA, Rao A, Haworth KB, Wen PY, Goldman S, Vezina G, MacDonald TJ, Dunkel IJ, Morgan PS, Jaspan T, Prados MD, Warren KE (2020) Response assessment in paediatric high-grade glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. Lancet Oncol 21(6):e317–e329. https://doi.org/10.1016/S1470-2045(20)30173-X
Craig E, Connolly DJ, Griffiths PD, Raghavan A, Lee V, Batty R (2012) MRI protocols for imaging paediatric brain tumours. Clin Radiol 67(9):829–832. https://doi.org/10.1016/j.crad.2012.03.018
Tejada S, Avula S, Pettorini B, Henningan D, Abernethy L, Mallucci C (2018) The impact of intraoperative magnetic resonance in routine pediatric neurosurgical practice-a 6-year appraisal. Childs Nerv Syst 34(4):617–626. https://doi.org/10.1007/s00381-018-3751-8
Choudhri AF, Klimo P Jr, Auschwitz TS, Whitehead MT, Boop FA (2014) 3T intraoperative MRI for management of pediatric CNS neoplasms. AJNR Am J Neuroradiol 35(12):2382–2387. https://doi.org/10.3174/ajnr.A4040
Avula S, Pettorini B, Abernethy L, Pizer B, Williams D, Mallucci C (2013) High field strength magnetic resonance imaging in paediatric brain tumour surgery--its role in prevention of early repeat resections. Childs Nerv Syst 29(10):1843–1850. https://doi.org/10.1007/s00381-013-2106-8
Roder C, Breitkopf M, Ms BS, Freitas Rda S, Dimostheni A, Ebinger M, Wolff M, Tatagiba M, Schuhmann MU (2016) Beneficial impact of high-field intraoperative magnetic resonance imaging on the efficacy of pediatric low-grade glioma surgery. Neurosurg Focus 40(3):E13. https://doi.org/10.3171/2015.11.focus15530
Knauth M, Aras N, Wirtz CR, Dorfler A, Engelhorn T, Sartor K (1999) Surgically induced intracranial contrast enhancement: potential source of diagnostic error in intraoperative MR imaging. AJNR Am J Neuroradiol 20(8):1547–1553
Avula S, Mallucci CL, Pizer B, Garlick D, Crooks D, Abernethy LJ (2012) Intraoperative 3-Tesla MRI in the management of paediatric cranial tumours--initial experience. Pediatr Radiol 42(2):158–167. https://doi.org/10.1007/s00247-011-2261-6
Yousaf J, Avula S, Abernethy LJ, Mallucci CL (2012) Importance of intraoperative magnetic resonance imaging for pediatric brain tumor surgery. Surg Neurol Int 3(Suppl 2):S65–S72. https://doi.org/10.4103/2152-7806.95417
Roder C, Haas P, Tatagiba M, Ernemann U, Bender B (2019) Technical limitations and pitfalls of diffusion-weighted imaging in intraoperative high-field MRI. Neurosurg Rev 44:327–334. https://doi.org/10.1007/s10143-019-01206-0
Smith JS, Cha S, Mayo MC, McDermott MW, Parsa AT, Chang SM, Dillon WP, Berger MS (2005) Serial diffusion-weighted magnetic resonance imaging in cases of glioma: distinguishing tumor recurrence from postresection injury. J Neurosurg 103(3):428–438. https://doi.org/10.3171/jns.2005.103.3.0428
Ozturk A, Oguz KK, Akalan N, Geyik PO, Cila A (2006) Evaluation of parenchymal changes at the operation site with early postoperative brain diffusion-weighted magnetic resonance imaging. Diagn Interv Radiol 12(3):115–120
Pala A, Brand C, Kapapa T, Hlavac M, König R, Schmitz B, Wirtz CR, Coburger J (2016) The value of intraoperative and early postoperative magnetic resonance imaging in low-grade glioma surgery: a retrospective study. World Neurosurg 93:191–197. https://doi.org/10.1016/j.wneu.2016.04.120
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Contributions
Shivaram Avula: Conceptualization, methodology, implementation, analysis, and writing—original draft
Tim Jaspan: Methodology, implementation, analysis, and writing—review and editing
Barry Pizer: Methodology, implementation, and writing—review and editing
Benedetta Pettorini: Methodology, implementation, and writing—review and editing
Deborah Garlick: Implementation and writing—review and editing
Dawn Hennigan: Implementation and writing—review and editing
Conor Mallucci: Methodology, implementation, and writing—review and editing
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
As the study involved comparison with standard clinical methodology and patients did not require additional general anaesthesia, it did not require consideration by the regional ethics committee and was registered as an institutional service evaluation study.
Informed consent
Patients/carers had consented for the anonymised scans to be used for the purpose of research and audit.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Avula, S., Jaspan, T., Pizer, B. et al. Comparison of intraoperative and post-operative 3-T MRI performed at 24–72 h following brain tumour resection in children. Neuroradiology 63, 1367–1376 (2021). https://doi.org/10.1007/s00234-021-02671-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-021-02671-5