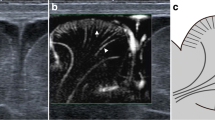
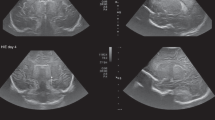
Abstract
Purpose
To explore the potential of superb microvascular imaging (SMI) in visualizing brain microvessels in preterm neonates of different gestational ages (GA).
Methods
In this retrospective, observational pilot study, 15 preterm newborns were equally divided into GA groups: extremely (GA < 28 weeks), very (28–31 weeks), and moderate to late (32–37 weeks) preterm. All patients underwent conventional transcranial ultrasounds during the first day of life following the American Institute of Ultrasound in Medicine practice guidelines. SMI was then performed; based on their SMI morphology and location, brain microvessels were classified as extrastriatal (cortical and medullary), striatal, or thalamic. Two examiners independently classified vessels as visible or invisible. To assess the association between vessel visibility and GA, binomial logistic regression analysis (separate for each microvessel group) was performed, taking visibility as a dependent variable and both examiners and GA as predictor variables.
Results
A statistically significant difference among GA groups was found in sex (P = 0.030), birth weight (P = 0.007), and Apgar score within 1 min after birth (P = 0.024). Microvascular visibility increased with GA for superficial vessels (P < 0.05 for both cortical and medullary), while striatal and thalamic vessels were visible in all neonates irrespective of their GA.
Conclusions
SMI technology shows promise to assess brain microvasculature in preterm neonates, even potentially providing data on early brain development.




Similar content being viewed by others
Abbreviations
- cSMI:
-
Color superb microvascular imaging
- EP:
-
Extremely preterm
- GA:
-
Gestational age
- IRR:
-
Inter-reader reliability
- MLP:
-
Moderate to late preterm
- MRI:
-
Magnetic resonance imaging
- mSMI:
-
Monochrome superb microvascular imaging
- SMI:
-
Superb microvascular imaging
- US:
-
Ultrasound
- VP:
-
Very preterm
References
Volpe J (2008) Neurology of the newborn, 5th edn. Elsevier Health Sciences, New York
Ecury-Goossen GM, Camfferman FA, Leijser LM, Govaert P, Dudink J (2015) State of the art cranial ultrasound imaging in neonates. J Vis Exp 96:e52238
Alderliesten T, Lemmers PMA, Smarius JJM, van de Vosse RE, Baerts W, van Bel F (2013) Cerebral oxygenation, extraction, and autoregulation in very preterm infants who develop peri-intraventricular hemorrhage. J Pediatr 162(4):698–704.e2
Perlman JM, McMenamin JB, Volpe JJ (1983) Fluctuating cerebral blood-flow velocity in respiratory-distress syndrome: relation to the development of intraventricular hemorrhage. N Engl J Med 309(4):204–209. https://doi.org/10.1056/NEJM198307283090402
Meek JH, Tyszczuk L, Elwell CE, Wyatt JS (1999) Low cerebral blood flow is a risk factor for severe intraventricular haemorrhage. Arch Dis Child Fetal Neonatal Ed 81(1):F15–F18. https://doi.org/10.1136/fn.81.1.F15
Perlman JM, Goodman S, Kreusser KL, Volpe JJ (1985) Reduction in intraventricular hemorrhage by elimination of fluctuating cerebral blood-flow velocity in preterm infants with respiratory distress syndrome. N Engl J Med 312(21):1353–1357
Ma Y, Li G, Li J, Ren WD (2015) The diagnostic value of superb microvascular imaging (SMI) in detecting blood flow signals of breast lesions: a preliminary study comparing SMI to color Doppler flow imaging. Medicine (Baltimore) 94(36):e1502
Xiao XY, Chen X, Guan XF, Wu H, Qin W, Luo BM (2016) Superb microvascular imaging in diagnosis of breast lesions: a comparative study with contrast-enhanced ultrasonographic microvascular imaging. Br J Radiol 89(1066):20160546
Machado P, Segal S, Lyshchik A, Forsberg F (2016) A novel microvascular flow technique: initial results in thyroids. UltrasoundQ 32(1):67–74
He M-N, Lv K, Jiang Y-X, Jiang T-A (2017) Application of superb microvascular imaging in focal liver lesions. World J Gastroenterol 23(43):7765–7775
Oura K, Kato T, Ohba H, Terayama Y (2018) Evaluation of intraplaque neovascularization using superb microvascular imaging and contrast-enhanced ultrasonography. J Stroke Cerebrovasc Dis 27(9):2348–2353
Cantisani V, David E, Ferrari D et al (2017) Color Doppler ultrasound with superb microvascular imaging compared to contrast enhanced ultrasound and computed tomography angiography to identify and classify endoleaks in patients undergoing EVAR. Ann Vasc Surg 40:136–145
Hasegawa J, Suzuki N (2016) SMI for imaging of placental infarction. Placenta 47:96–98
Hata T, Kanenishi K, Yamamoto K, Abo Ellail MAM, Mashima M, Mori N (2018) Microvascular imaging of thick placenta with fetal growth restriction. Ultrasound Obstet Gynecol 51(6):837–839
Kim HK, O’Hara S, Je BK, Kraus SJ, Horn P (2018) Feasibility of superb microvascular imaging to detect high-grade vesicoureteral reflux in children with urinary tract infection. EurRadiol 28(1):66–73
Karaca L, Oral A, Kantarci M et al (2016) Comparison of the superb microvascular imaging technique and the color Doppler techniques for evaluating children’s testicular blood flow. Eur Rev Med Pharmacol Sci 20(10):1947–1953
Lee YS, Kim MJ, Han SW et al (2016) Superb microvascular imaging for the detection of parenchymal perfusion in normal and undescended testes in young children. Eur J Radiol 85(3):649–656
Goeral K, Hojreh A, Kasprian G et al (2019) Microvessel ultrasound of neonatal brain parenchyma: feasibility, reproducibility, and normal imaging features by superb microvascular imaging (SMI). Eur Radiol 29:2127–2136
Howson CP, Kinney MV, Lawn JE (2012) Born too soon: the global action report on preterm birth. March of Dimes, PMNCH. In: Save the children. WHO World Health Organization, Geneva
American Institute of Ultrasound in Medicine (AIUM), American College of Radiology (ACR), Society of Radiologists in Ultrasound (SRU) (2014) AIUM practice guideline for the performance of neurosonography in neonates and infants. J Ultrasound Med 33(6):1103–1110
van Wezel-Meijler G, Steggerda SJ, Leijser LM (2010) Cranial ultrasonography in neonates: role and limitations. Semin Perinatol 34:28–384
Hallgren KA (2012) Computing inter-rater reliability for observational data: an overview and tutorial. Tutor Quant Methods Psychol 8(1):23–34
Tortora D, Severino M, Malova M et al (2018) Differences in subependymal vein anatomy may predispose preterm infants to GMH-IVH. Arch Dis Child Fetal Neonatal Ed 103(1):F59–F65
Tortora D, Severino M, Malova M et al (2016) Variability of cerebral deep venous system in preterm and term neonates evaluated on MR SWI venography. AJNR Am J Neuroradiol 37(11):2144–2149
Wigglesworth JS, Pape KE (1978) An integrated model for haemorrhagic and ischaemic lesions in the newborn brain. Early Hum Dev 2(2):179–199
Haruda FD (2001) The structure of blood vessels in the germinal matrix and the autoregulation of cerebral blood flow in premature infants. Pediatrics 106(4):625–632
Mahdi ES, Bouyssi-Kobar M, Jacobs MB, Murnick J, Chang T, Limperopoulos C (2018) Cerebral perfusion is perturbed by preterm birth and brain injury. AJNR Am J Neuroradiol 39(7):1330–1335
American Academy of Pediatrics Committee on Fetus and Newborn; American College of Obstetricians and Gynecologists Committee on Obstetric Practice (2015) The Apgar score Pediatrics 136(4):819–822
American Academy of Pediatrics, Committee on Fetus and Newborn; American College of Obstetricians and Gynecologists and Committee on Obstetric Practice (2006) The Apgar score. Pediatrics 117(4):1444–1447
Catlin EA, Carpenter MW, BSt B et al (1986) The Apgar score revisited: influence of gestational age. J Pediatr 109(5):865–868
Weinberger B, Anwar M, Hegyi T, Hiatt M, Koons A, Paneth N (2000) Antecedents and neonatal consequences of low Apgar scores in preterm newborns: a population study. Arch Pediatr Adolesc Med 154(3):294–300
Raybaud C (2010) Normal and abnormal embryology and development of the intracranial vascular system. Neurosurg Clin N Am 21(3):399–426
Kuban KC, Gilles FH (1985) Human telencephalic angiogenesis. Ann Neurol 17(6):539–548
Norman MG, O’Kusky JR (1986) The growth and development of microvasculature in human cerebral cortex. J Neuropathol Exp Neurol 45(3):222–232
Funding
No funding was received for this study.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in the studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Keypoints
- Superb microvascular imaging (SMI) provides a noninvasive tool for assessing brain microvessels in preterm neonates, potentially supporting the diagnosis of brain vascular disorders and monitoring of normal brain development.
- Cortical and medullary vessel visibility increases with gestational age (GA) (P < 0.05), while striatal and thalamic vessels are visible irrespective of GA.
Supplementary information
ESM 1
(PDF 296 kb)
Rights and permissions
About this article
Cite this article
Barletta, A., Balbi, M., Surace, A. et al. Cerebral superb microvascular imaging in preterm neonates: in vivo evaluation of thalamic, striatal, and extrastriatal angioarchitecture. Neuroradiology 63, 1103–1112 (2021). https://doi.org/10.1007/s00234-021-02634-w
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-021-02634-w