Abstract
Purpose
The optimum strategy for the surveillance of low-grade gliomas in children has not been established, and there is concern about the use of gadolinium-based contrast agents (GBCAs), particularly in children, due to their deposition in the brain. The number of surveillance scans and the use of GBCAs in surveillance of low-risk tumours should ideally be limited. We aimed to investigate the consistency and utility of our surveillance imaging and also determine to what extent the use of GBCAs contributed to decisions to escalate treatment in children with grade 1 astrocytomas.
Methods
This was a retrospective single-centre study at a tertiary paediatric hospital. All children with a new diagnosis of a non-syndromic World Health Organization (WHO) grade 1 astrocytoma between 2007 and 2013 were included, with surveillance imaging up to December 2018 included in analysis. The intervals of surveillance imaging were recorded, and imaging and electronic health records were examined for decisions related to treatment escalation.
Results
Eighty-eight patients had 690 surveillance scans in the study period. Thirty-one patients had recurrence or progression leading to treatment escalation, 30 of whom were identified on surveillance imaging. The use of GBCAs did not appear to contribute to multidisciplinary team (MDT) decisions in the majority of cases.
Conclusion
Surveillance imaging could be reduced in number and duration for completely resected cerebellar tumours. MDT decisions were rarely made on the basis of post-contrast imaging, and GBCA administration could therefore potentially be restricted in the setting of surveillance of grade 1 astrocytomas in children.
Similar content being viewed by others
Avoid common mistakes on your manuscript.
Introduction
Surveillance imaging in children with low-grade brain tumours, who can comprise up to 40% of all children with brain tumours, presents an important clinical dilemma, and one for which there are not currently any published guidelines derived from high-quality evidence. It has been demonstrated that recurrence or progression of low-grade paediatric brain tumours can occur in the absence of clinical symptoms [1], necessitating regular scheduled imaging follow-up to permit a prompt treatment intervention given the superior surgical resection potential and subsequent survival benefit in early relapse or progression. However, surveillance imaging is not without cost for all involved, particularly for young children where repeat magnetic resonance imaging (MRI) can be distressing or involve sedation or anaesthesia with their own inherent risks, and in the current climate with the COVID-19 pandemic, additional hospital attendances should be minimised where possible. This is in addition to the cost of anxiety for the family surrounding every scan performed and the time and workload burden for clinicians. The evidence available in the literature to date is limited and does not convincingly support any particular surveillance strategy [2], and as such there is no consensus approach as to the frequency or duration of surveillance imaging, resulting in a lack of heterogeneous practice even within hospitals, let alone nations. This is not satisfactory patient care.
Furthermore, there has been recent concern over the deposition of gadolinium in the brain following repeated administration of gadolinium-based contrast agents [3]. This has been demonstrated on both imaging and post-mortem examination in children [4] and although no harm has yet been demonstrated as a result, this requires consideration as a potential risk when planning surveillance imaging, particularly as, given the generally favourable prognosis of these tumours, toxicity from treatment and by implication surveillance should be minimised [5]. Clearly, however, this must be balanced against the currently greater risk of delayed diagnosis of progressive or recurrent disease.
In this single-centre retrospective study at a tertiary paediatric hospital, we examined our large cohort of patients with grade 1 astrocytomas to assess the asymptomatic recurrence rate and determine if a more optimised distribution of surveillance imaging could be universally applied. A secondary objective was to assess the impact of gadolinium administration on treatment decisions based on surveillance imaging.
Methods
This single-centre retrospective study was registered as an audit against the ICLGG/SIOP Cooperative Multicenter Study for Children and Adolescents with Low Grade Glioma ‘Guidance for surveillance imaging in paediatric low grade gliomas’, described by Gnekow et al. [6], as a benchmark against which to measure our institutional practice. Our standard imaging protocol for follow-up of these patients includes an axial T2-weighted sequence, a coronal fluid attenuated inversion recovery (FLAIR) sequence, diffusion-weighted imaging (DWI), susceptibility weighted imaging (SWI), and volumetric T1-weighted sequences before and after the administration of GBCA.
Patients were identified by interrogation of our prospectively maintained neuro-oncology centre database for paediatric patients (below the age of 16) with a new diagnosis of a WHO classified grade I astrocytoma diagnosed between January 2007 and December 2013 (to ensure at least 5 years of follow-up). All tumour locations were included, and all patients with a histologically confirmed low-grade astrocytoma or those without a histological diagnosis but with no high-grade features on follow-up imaging were also included (for example optic pathway tumours). Patients without at least two year’s local follow-up available for analysis were excluded, and patients with a tumour syndrome (such as neurofibromatosis type 1 or tuberous sclerosis) were excluded.
For each patient, age at diagnosis, treatment summary (including extent of resection if tumour surgically excised), histological diagnosis, and location of tumour were recorded. The index imaging, postoperative imaging findings, and subsequent number of MRI scans were also recorded, with documentation of the interval between surveillance scans, as well as any additional MRI studies performed in response to new symptoms. For every change (confirmed or suspected) in tumour appearance on the surveillance imaging, the relevant neuro-oncology multidisciplinary team meeting notes or clinic letters were scrutinised for evidence of a consequent change in management.
We used the same definition of a surveillance MRI as in a previous study from our centre [7]: an MRI performed for the purpose of monitoring a low-grade astrocytoma in the absence of any clinical evidence indicating further tumour growth, with the first postoperative MRI not considered to represent surveillance.
Determination of extent of resection was made based on the first postoperative imaging study, usually performed within 48 h of surgery, and categorised as either complete or incomplete resection.
A change in clinical management following surveillance imaging was considered to include institution of a new therapy or surgical intervention. Recurrence or progression was defined as a change in imaging features on surveillance imaging that led to a change in clinical management. Our institutional clinical practice is not to use tumour volume measurements as outlined in trial criteria to define progression, but to evaluate patients on a case by case basis; this is necessarily subjective which is a potential limitation of the current study. For cases of recurrence or progression, the report and the imaging were scrutinised to determine whether the administration of gadolinium-based contrast agent contributed to the consequent management decision.
Results
In total, 88 patients were included for analysis, with a mean age of 7 ± 5 years (range 1 to 15). No patients included had neurofibromatosis type one. The patient cohort were divided by tumour location for analysis, with 42 (48%) patients having cerebellar tumours (cerebellar group), 20 (23%) patients having optic pathway tumours (OP group), and 26 (29%) patients with tumours which were in neither of these locations (‘Other Group’; included thalamic, midbrain, and lobar non-optic pathway lesions). Sixty-seven of 88 had a histological diagnosis, all of which were WHO grade 1 tumours; these included 3 pleomorphic xanthoastrocytomas, 1 angiocentric glioma, and 63 pilocytic astrocytomas.
Audit of our local surveillance imaging practice showed heterogeneity amongst patients and clinicians; the median interval between imaging was between 4 and 6 months for the first 8 surveillance studies but the standard deviation was also 4 to 6 months. In total, 690 surveillance scans were performed over the study period.
Sixty-two of 88 (70%) patients had surgery as initial management, with 33/62 (53%) having had a complete resection. Thirty-one of 88 (35%) tumours recurred or progressed in the study period examined. Thirty of these (97%) were detected by surveillance imaging (i.e. the child was asymptomatic at the time of the surveillance imaging examination considered to demonstrate progression leading to a change in clinical management). This gave a detection rate per surveillance MRI of 30/690 (4%). The timings of each recurrence are demonstrated in Fig. 1.
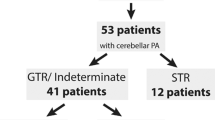
In the cerebellar group, 40/42 (95%) patients underwent surgical tumour resection, 25/40 (63%) achieved complete resection, and 15/40 (37%) incomplete resection. Throughout the study period, 1/25 (4%) patients with a complete initial surgical resection had recurrence at 13 months, and 7/15 (47%) patients having had an incomplete resection demonstrated tumour progression. The mean duration to progression was 26 months, range 4–46 months.
In the optic pathway group, 5/20 (25%) tumours had surgical resection, 1 (20%) had a complete resection, and 4 (80%) had incomplete resection. Throughout the study period, 12/20 (60%) patients demonstrated tumour progression, 3 (25%) of which were subsequent to an incomplete surgical resection or tumour debulking. The mean duration to progression was 34 months, range 21–78 months.
In the ‘other’ group, 17/26 (65%) tumours were surgically resected, 7/17 (41%) patients had a complete resection, and 10/17 having incomplete resection (59%). Throughout the study period, 11/26 (42%) patients had tumour recurrence or progression: 6 of the 10 (60%) having had incomplete resection and 3/7 (43%) having had a complete resection. The mean duration to tumour progression or relapse was 28 months, range 14–64 months.
In total, 13/690 (2%) surveillance MRI scans demonstrated varying degrees of contrast enhancement throughout the study period without increasing signal abnormality on other sequences; 5 (38%) of these represented linear enhancement following surgery which was considered to be postoperative. We found no cases of recurrent disseminated disease. In the absence of associated progression in the degree of signal abnormality on other sequences (T2, FLAIR, or DWI primarily), no escalation of treatment was undertaken on the basis of increased enhancement alone.
Discussion
This retrospective single-centre review investigates the schedule of and intervals between surveillance scanning of children with presumed or confirmed WHO grade 1 gliomas. In addition, we investigated the duration to tumour recurrence or progression in the context of attempting to identify whether reducing the number of surveillance MRI scans, along with increasing the interval between such scans, would permit a balance between identifying tumour relapse or progression requiring intervention with the burden and risks of performing excessive surveillance imaging.
Even from our single centre, the results demonstrate significant variation in clinical practice as to the approach to surveillance MRI imaging, with varying number of and interval between surveillance scans. Our data suggests that the default interval between MRI scans is 6 months (which is in keeping with the SIOP-E-BTG guidance), although this varied depending on tumour location and degree of surgical resection. However, the large number of surveillance scans compared to the relatively small number of instances of recurrence/progression suggest that there may be scope to reduce the volume and frequency of surveillance imaging.
Our results support previous published evidence demonstrating that completely resected cerebellar grade 1 astrocytomas rarely recur [2], with only one of 25 (4%) patients in our cohort recurring. For this group of patients in particular, we therefore suggest that a reduced frequency of surveillance imaging and a shorter overall surveillance period is likely to be adequate and to achieve a better balance for patients. McAuley et al. suggest a protocol for completely resected cerebellar astrocytomas of imaging at 6, 18, and 30 months [8]. In our cohort, this would have led to a delayed diagnosis in our single recurrence at 13 months but whether this would have changed outcome is difficult to determine retrospectively. However, the absence of recurrence at longer intervals suggests that it would be prudent to limit the overall surveillance period and McAuley’s suggestion of 30 months seems reasonable according to our data.
There is very limited data regarding the optimum surveillance imaging schedule in non-cerebellar tumours, as well as in tumours that are non-operatively managed or incompletely resected. Stevens et al. highlight that 75% of the tumours in their systematic review of surveillance imaging were cerebellar and the majority completely resected (which is likely to explain why our recurrence/progression rate of 35% is higher than their reported overall rate of 24%) [2].
The progressions that occurred furthest from resection were in an optic pathway tumour which had been debulked and received chemotherapy, at 78 months, and a tectal plate tumour (not histologically confirmed but considered to be grade 1 based on imaging and treated with chemotherapy) at 64 months but the remaining cases of progression or recurrence occurred within 4 years. Kim et al. suggest an imaging strategy of MRI at 3 months and 1, 2, 5, and 10 years [1]. In the context of incomplete resection or non-operative management, it may not be possible to conclusively determine when surveillance imaging should be discontinued. Our results would appear to favour a lengthened interval—imaging at 6 and then 12 months and then annually up to 5 years would have reduced the number of MRI scans whilst minimising the delay to diagnosis for the majority of patients in our cohort. However, until further cohorts support these findings, we feel it is prudent to maintain 6 monthly imaging surveillance for non-cerebellar (or incompletely resected cerebellar) tumours currently.
A more recent study examined the detection efficacy and cost of surveillance imaging in a retrospective analysis of 517 patients with low-grade gliomas using an algorithmic approach to define a more cost-effective surveillance programme [9]. This is an interesting and useful approach, but no distinction was made between the location of the tumours and our data suggests there is a difference in recurrence/progression risk depending on the location of the tumour and as such the generalisability is difficult to determine.
Overall, our data would suggest that the available guidelines are fairly arbitrary and lead to a very low detection rate with a large burden on the patient, family, and hospital. Multiple studies suggest a shorter and less frequent surveillance protocol; we would suggest based on these findings that this should be tailored to the location of the tumour as well as the degree of initial resection (where relevant), and that the protocol outlined by McAuley et al. [8] seems most appropriate for cerebellar tumours and would have avoided missing instances of recurrence or progression in our cohort. However, more data is required on surveillance patterns in non-cerebellar tumours.
The use of gadolinium-based contrast agents (GBCAs) in surveillance imaging has become more controversial given the recent published demonstration of deposition of gadolinium in the brain. It has previously been suggested that post-contrast T1 is the most sensitive sequence for tumour recurrence [10]. However, in our cohort of 13 patients with an isolated change in contrast enhancement, this justified discussion in the neuro-oncology MDT meeting but was never sufficient alone to indicate treatment escalation without other symptoms or evidence of tumour progression.
This finding is echoed by recent published evidence specifically examining low-grade optic pathway tumours. Maloney et al. report that in their cohort of children with isolated optic pathway gliomas, treatment escalation was only instigated in the context of increased contrast enhancement with concomitant increase in tumour size visible on T2-weighted images, and thus propose use of a non-contrast surveillance protocol [11]. An unenhanced surveillance protocol has subsequently been shown by Marsault et al. to have satisfactory sensitivity and specificity, based primarily on assessment of tumour volume variation [12]. We believe that the use of GBCAs could be limited in the setting of all low-grade paediatric astrocytomas but this requires further investigation in other tumour locations, as performing repeat MRI surveillance scans for GBCA administration is not ethical when it often involves risk of sedation or anaesthesia.
We note that the recent Response Assessment in Paediatric Neuro-Oncology (RAPNO) working group recommendations highlight the evidence that contrast enhancement may be less reliable than T2 signal in assessing response, but currently recommend it as a core sequence for follow-up [13]. It is also important to note the additional value of GBCAs in detecting disseminated disease at recurrence, which if not nodular may be difficult to identify on unenhanced imaging. The risk of this in low-grade tumours is relatively small, but not insignificant and is higher in infants. In our cohort, there was no dissemination of disease at recurrence (including on post-contrast imaging), but we note previous studies demonstrate a 4.3% incidence of dissemination in all low-grade gliomas [14] and increased incidence in infants under one [15].
There are limitations to the conclusions made from interpretation of our retrospective dataset. Firstly, we have included multiple tumours not histologically confirmed as grade 1 astrocytomas. This was deemed necessary given that optic pathway gliomas are often not amenable to surgical access for biopsy or resection and yet fall within the surveillance purview of low-grade gliomas. Despite minimising the risk of confounding results by excluding any tumours with high-grade radiological features, in the absence of a tissue diagnosis, we cannot confidently conclude that all patients included did have low-grade tumours.
Secondly, the variation in treatment and management options for non-cerebellar astrocytomas (with respect to surgical debulking or resection, chemotherapy, and radiotherapy options) means that the surveillance data for non-cerebellar tumours is more challenging to objectively analyse, and therefore only limited conclusions can be drawn from the data as a whole. The retrospective methodology may have limited our data analysis, with variable patient follow-up durations as many of our patients transferred to adult services or alternate regional hospitals closer to home throughout the surveillance period. Finally, our interpretation of the conclusions made at multidisciplinary team meetings is somewhat subjective, particularly with regard to the utility of GBCAs, and this should therefore be interpreted with caution. On the other hand, it could be deemed that multiple oncologists and neuro-radiologists concluding non-tumour progression in the presence of progressive contrast enhancement strengthens our conclusions.
Conclusion
Surveillance imaging of paediatric grade 1 astrocytomas continues to represent a difficult clinical challenge for which there is limited objective and translatable evidence. Our single-centre experience suggests that follow-up surveillance for completely resected cerebellar astrocytomas can be reduced. In addition, we may be able to reduce the frequency of surveillance imaging of non-cerebellar grade 1 astrocytomas, but there is insufficient evidence from this review and other published data to curtail the duration of surveillance given the incidence of ‘late’ recurrence or progressions. Finally, there is subjective evidence to propose that we may be able to limit the use of GBCAs without loss of efficacy of surveillance, but this requires further investigation in larger, multi-centre studies and consideration of the risk of missing disseminated disease.
References
Kim AH, Thompson EA, Governale LS, Santa C, Cahll K, Kieran MW, Chi SN, Ullrich NJ, Scott RM, Goumnerova LC (2014) Recurrence after gross-total resection of low-grade pediatric brain tumors: the frequency and timing of postoperative imaging. J Neurosurg Pediatr 14(4):356–364. https://doi.org/10.3171/2014.6.peds1321
Stevens SP, Main C, Bailey S, Pizer B, English M, Phillips R, Peet A, Avula S, Wilne S, Wheatley K, Kearns PR, Wilson JS (2018) The utility of routine surveillance screening with magnetic resonance imaging (MRI) to detect tumour recurrence in children with low-grade central nervous system (CNS) tumours: a systematic review. J Neurooncol 139(3):507–522. https://doi.org/10.1007/s11060-018-2901-x
Guo BJ, Yang ZL, Zhang LJ (2018) Gadolinium deposition in brain: current scientific evidence and future perspectives, (in eng). Front Mol Neurosci, vol. 11, doi: https://doi.org/10.3389/fnmol.2018.00335
McDonald JS et al (2017) Intracranial gadolinium deposition following gadodiamide-enhanced magnetic resonance imaging in pediatric patients: a case-control study. JAMA Pediatr 171(7):705–707. https://doi.org/10.1001/jamapediatrics.2017.0264
Bandopadhayay P, Bergthold G, London WB, Goumnerova LC, Morales la Madrid A, Marcus KJ, Guo D, Ullrich NJ, Robison NJ, Chi SN, Beroukhim R, Kieran MW, Manley PE (2014) Long-term outcome of 4,040 children diagnosed with pediatric low-grade gliomas: an analysis of the Surveillance Epidemiology and End Results (SEER) database. Pediatr Blood Cancer 61(7):1173–1179. https://doi.org/10.1002/pbc.24958
Gnekow AK et al (2019) SIOP-E-BTG and GPOH guidelines for diagnosis and treatment of children and adolescents with low grade glioma. Klin Padiatr 231(3):107–135. https://doi.org/10.1055/a-0889-8256
Saunders DE, Phipps KP, Wade AM, Hayward RD (2005) Surveillance imaging strategies following surgery and/or radiotherapy for childhood cerebellar low-grade astrocytoma. J Neurosurg 102(2 Suppl):172–178. https://doi.org/10.3171/jns.2005.102.2.0172
McAuley E, Brophy H, Hayden J, Pettorini B, Parks C, Avula S, Mallucci C, Pizer B (2019) The benefit of surveillance imaging for paediatric cerebellar pilocytic astrocytoma. Childs Nerv Syst 35(5):801–805. https://doi.org/10.1007/s00381-019-04078-3
Zaazoue MA et al. (2019) Optimizing postoperative surveillance of pediatric low-grade glioma using tumor behavior patterns, Neurosurgery, https://doi.org/10.1093/neuros/nyz072
Udaka YT, Yeh-Nayre LA, Amene CS, VandenBerg SR, Levy ML, Crawford JR (2013) Recurrent pediatric central nervous system low-grade gliomas: the role of surveillance neuroimaging in asymptomatic children. J Neurosurg Pediatr 11(2):119–126. https://doi.org/10.3171/2012.10.peds12307
Maloney E, Stanescu AL, Perez FA, Iyer RS, Otto RK, Leary S, Steuten L, Phipps AI, Shaw DWW (2018) Surveillance magnetic resonance imaging for isolated optic pathway gliomas: is gadolinium necessary? Pediatr Radiol 48(10):1472–1484. https://doi.org/10.1007/s00247-018-4154-4
Marsault P, Ducassou S, Menut F, Bessou P, Havez-Enjolras M, Chateil JF (2019) Diagnostic performance of an unenhanced MRI exam for tumor follow-up of the optic pathway gliomas in children. Neuroradiology 61(6):711–720. https://doi.org/10.1007/s00234-019-02198-w
Erker C, Tamrazi B, Poussaint TY, Mueller S, Mata-Mbemba D, Franceschi E, Brandes AA, Rao A, Haworth KB, Wen PY, Goldman S, Vezina G, MacDonald TJ, Dunkel IJ, Morgan PS, Jaspan T, Prados MD, Warren KE Response assessment in paediatric high-grade glioma: recommendations from the Response Assessment in Pediatric Neuro-Oncology (RAPNO) working group. The Lancet Oncology 21(6):2020–e329. https://doi.org/10.1016/S1470-2045(20)30173-X
Gnekow A et al (2012) Long-term follow-up of the multicenter, multidisciplinary treatment study HIT-LGG-1996 for low-grade glioma in children and adolescents of the German Speaking Society of Pediatric Oncology and Hematology. Neuro-Oncology 14(10):1265–1284. https://doi.org/10.1093/neuonc/nos202
M C et al (2014) Children <1 year show an inferior outcome when treated according to the traditional LGG treatment strategy: a report from the German multicenter trial HIT-LGG 1996 for children with low grade glioma (LGG). Pediatric Blood & Cancer 61(3):457–463. https://doi.org/10.1002/pbc.24729
Acknowledgments
Please note that preliminary data from this study was presented at the European Society of Neuroradiology Annual Meeting in 2019, the abstract of which was published in Neuroradiology (Neuroradiology 61, 1-137 (2019)).
Availability of data and material
Not available.
Funding
No funding specific to this project was received. However, all research at Great Ormond Street Hospital Foundation Trust and the Institute of Child Health is made possible by the NIHR Great Ormond Street Hospital Biomedical Research Centre. The views expressed are those of the authors and not necessarily those of the NHS, the NIHR, or the Department of Health.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflicts of interest/competing interests
Darren Hargrave is involved with several consultancies for novel therapies for paediatric low-grade glioma as listed below but these are not the subject of this manuscript: AstraZeneca (selumetinib), Novartis (trametinib, dabrafenib), Roche (Bevacizumab, cobimetinib).
No declarations by the other authors.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards. It was approved by the Clinical Audit department at Great Ormond Street Hospital (ref 2460).
Consent to participate
For this type of study, formal consent is not required; this was a retrospective study which was approved by the clinical audit department.
Code availability
Not applicable.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Campion, T., Quirk, B., Cooper, J. et al. Surveillance imaging of grade 1 astrocytomas in children: can duration and frequency of follow-up imaging and the use of contrast agents be reduced?. Neuroradiology 63, 953–958 (2021). https://doi.org/10.1007/s00234-020-02609-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-020-02609-3