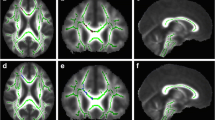
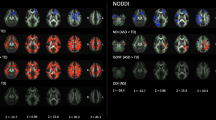
Abstract
Purpose
To investigate the gross white matter abnormalities in the structural brain MR imaging as well as white matter microstructural alterations using tract-based spatial statistics (TBSS) analysis of diffusion tensor imaging (DTI) in both affected and contralateral cerebral hemispheres of children with hemimegalencephaly (HMEG).
Methods
From 2003 to 2019, we retrospectively reviewed brain MR images in 20 children (11 boys, 2 days–16.5 years) with HMEG, focusing on gross white matter abnormalities. DTI was evaluated in 12 patients (8 boys, 3 months–16.5 years) with HMEG and 12 age-, sex-, and magnetic field strength-matched control subjects. TBSS analysis was performed to analyze main white matter tracts. Regions of significant differences in fractional anisotropy (FA) were determined between HMEG and control subjects and between affected and contralateral hemispheres of HMEG.
Results
Gross white matter abnormalities were noted in both affected (n = 20, 100%) and contralateral hemisphere (n = 4, 20%) of HMEG. FA values were significantly decreased in both hemispheres of HMEG, compared with control subjects (P < 0.05). Contralateral hemispheres of HMEG showed regions with significantly decreased FA values compared with affected hemispheres (P < 0.05).
Conclusions
In addition to gross white matter abnormalities particularly evident in affected hemispheres, DTI analysis detected widespread microstructural alterations in both affected and contralateral hemispheres in HMEG suggesting HMEG may involve broader abnormalities in neuronal networks.



Similar content being viewed by others
Abbreviations
- HMEG:
-
Hemimegalencephaly
- DTI:
-
Diffusion tensor imaging
- FA:
-
Fractional anisotropy
- ROI:
-
Region-of-interest
- TBSS:
-
Tract-based spatial statistics
- MNI:
-
Montreal Neurological Institute
- FSL:
-
FMRIB Software Library
- mTOR:
-
Mammalian target of rapamycin
References
Christensen J, Pedersen MG, Pedersen CB, Sidenius P, Olsen J, Vestergaard M (2009) Long-term risk of epilepsy after traumatic brain injury in children and young adults: a population-based cohort study. Lancet 373:1105–1110
Fitz CR, Harwood-Nash DC, Boldt DW (1978) The radiographic features of unilateral megalencephaly. Neuroradiology 15:145–148
Yagishita A, Arai N, Tamagawa K, Oda M (1998) Hemimegalencephaly: signal changes suggesting abnormal myelination on MRI. Neuroradiology 40:734–738
Kato M, Mizuguchi M, Sakuta R, Takashima S (1996) Hypertrophy of the cerebral white matter in hemimegalencephaly. Pediatr Neurol 14:335–338
Kamiya K, Sato N, Saito Y, Nakata Y, Ito K, Shigemoto Y, Ota M, Sasaki M, Ohtomo K (2014) Accelerated myelination along fiber tracts in patients with hemimegalencephaly. J Neuroradiol 41:202–210
Uematsu M, Haginoya K, Togashi N, Hino-Fukuyo N, Nakayama T, Kikuchi A, Abe Y, Wakusawa K, Matsumoto Y, Kakisaka Y, Kobayashi T, Hirose M, Yokoyama H, Iinuma K, Iwasaki M, Nakasato N, Kaneta T, Akasaka M, Kamei A, Tsuchiya S (2010) Unique discrepancy between cerebral blood flow and glucose metabolism in hemimegalencephaly. Epilepsy Res 92:201–208
Salamon N, Andres M, Chute DJ, Nguyen ST, Chang JW, Huynh MN, Chandra PS, Andre VM, Cepeda C, Levine MS, Leite JP, Neder L, Vinters HV, Mathern GW (2006) Contralateral hemimicrencephaly and clinical-pathological correlations in children with hemimegalencephaly. Brain 129:352–365
Shiroishi MS, Jackson HA, Nelson MD Jr, Bluml S, Panigrahy A (2010) Contralateral hemimicrencephaly in neonatal hemimegalencephaly. Pediatr Radiol 40:1826–1830
Le Bihan D (2003) Looking into the functional architecture of the brain with diffusion MRI. Nat Rev Neurosci 4:469–480
Winston GP (2012) The physical and biological basis of quantitative parameters derived from diffusion MRI. Quant Imaging Med Surg 2:254–265
Sato N, Ota M, Yagishita A, Miki Y, Takahashi T, Adachi Y, Nakata Y, Sugai K, Sasaki M (2008) Aberrant midsagittal fiber tracts in patients with hemimegalencephaly. AJNR Am J Neuroradiol 29:823–827
Takahashi T, Sato N, Ota M, Nakata Y, Yamashita F, Adachi Y, Saito Y, Sugai K, Sasaki M, Asada T (2009) Asymmetrical interhemispheric fiber tracts in patients with hemimegalencephaly on diffusion tensor magnetic resonance imaging. J Neuroradiol 36:249–254
Oikawa T, Tatewaki Y, Murata T, Kato Y, Mugikura S, Takase K, Takahashi S (2015) Utility of diffusion tensor imaging parameters for diagnosis of hemimegalencephaly. Neuroradiol J 28:628–633
Re TJ, Scarciolla L, Takahashi E, Specchio N, Bernardi B, Longo D (2015) Magnetic resonance fiber tracking in a neonate with hemimegalencephaly. J Neuroimaging 25:844–847
Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE (2006) Tract-based spatial statistics: voxelwise analysis of multi-subject diffusion data. Neuroimage 31:1487–1505
Dubois J, Dehaene-Lambertz G, Kulikova S, Poupon C, Huppi PS, Hertz-Pannier L (2014) The early development of brain white matter: a review of imaging studies in fetuses, newborns and infants. Neuroscience 276:48–71
Flores-Sarnat L (2002) Hemimegalencephaly: part 1. Genetic, clinical, and imaging aspects. J Child Neurol 17:373–384 discussion 384
Sato N, Yagishita A, Oba H, Miki Y, Nakata Y, Yamashita F, Nemoto K, Sugai K, Sasaki M (2007) Hemimegalencephaly: a study of abnormalities occurring outside the involved hemisphere. AJNR Am J Neuroradiol 28:678–682
Takashima S, Chan F, Becker LE, Kuruta H (1991) Aberrant neuronal development in hemimegalencephaly: immunohistochemical and Golgi studies. Pediatr Neurol 7:275–280
Sarnat H, Flores-Sarnat L, Crino P, Hader W, Bello-Espinosa L (2012) Hemimegalencephaly: foetal tauopathy with mTOR hyperactivation and neuronal lipidosis. Folia Neuropathol 50:330–345
Soufflet C, Bulteau C, Delalande O, Pinton F, Jalin C, Plouin P, Bahi-Buisson N, Dulac O, Chiron C (2004) The nonmalformed hemisphere is secondarily impaired in young children with hemimegalencephaly: a pre- and postsurgery study with SPECT and EEG. Epilepsia 45:1375–1382
Hanefeld F, Kruse B, Holzbach U, Christen HJ, Merboldt KD, Hanicke W, Frahm J (1995) Hemimegalencephaly: localized proton magnetic resonance spectroscopy in vivo. Epilepsia 36:1215–1224
Rintahaka PJ, Chugani HT, Messa C, Phelps ME (1993) Hemimegalencephaly: evaluation with positron emission tomography. Pediatr Neurol 9:21–28
Jahan R, Mischel PS, Curran JG, Peacock WJ, Shields DW, Vinters HV (1997) Bilateral neuropathologic changes in a child with hemimegalencephaly. Pediatr Neurol 17:344–349
Lee JH, Huynh M, Silhavy JL, Kim S, Dixon-Salazar T, Heiberg A, Scott E, Bafna V, Hill KJ, Collazo A, Funari V, Russ C, Gabriel SB, Mathern GW, Gleeson JG (2012) De novo somatic mutations in components of the PI3K-AKT3-mTOR pathway cause hemimegalencephaly. Nat Genet 44:941–945
Pelorosso C, Watrin F, Conti V, Buhler E, Gelot A, Yang X, Mei D, McEvoy-Venneri J, Manent JB, Cetica V, Ball LL, Buccoliero AM, Vinck A, Barba C, Gleeson JG, Guerrini R, Represa A (2019) Somatic double-hit in MTOR and RPS6 in hemimegalencephaly with intractable epilepsy. Hum Mol Genet 28:3755–3765
D’Gama AM, Geng Y, Couto JA, Martin B, Boyle EA, LaCoursiere CM, Hossain A, Hatem NE, Barry BJ, Kwiatkowski DJ, Vinters HV, Barkovich AJ, Shendure J, Mathern GW, Walsh CA, Poduri A (2015) Mammalian target of rapamycin pathway mutations cause hemimegalencephaly and focal cortical dysplasia. Ann Neurol 77:720–725
Enokizono M, Sato N, Ota M, Shigemoto Y, Morimoto E, Oba M, Sone D, Kimura Y, Sugai K, Sasaki M, Ikegaya N, Iwasaki M, Matsuda H (2019) Disrupted cortico-ponto-cerebellar pathway in patients with hemimegalencephaly. Brain and Development 41:507–515
Salcman M, Defendini R, Correll J, Gilman S (1978) Neuropathological changes in cerebellar biopsies of epileptic patients. Ann Neurol 3:10–19
Boer K, Troost D, Spliet WG, Redeker S, Crino PB, Aronica E (2007) A neuropathological study of two autopsy cases of syndromic hemimegalencephaly. Neuropathol Appl Neurobiol 33:455–470
Ly MT, Nanavati TU, Frum CA, Pergami P (2015) Comparing tract-based spatial statistics and manual region-of-interest labeling as diffusion analysis methods to detect white matter abnormalities in infants with hypoxic-ischemic encephalopathy. J Magn Reson Imaging 42:1689–1697
Huisman TA, Loenneker T, Barta G, Bellemann ME, Hennig J, Fischer JE, Il’yasov KA (2006) Quantitative diffusion tensor MR imaging of the brain: field strength related variance of apparent diffusion coefficient (ADC) and fractional anisotropy (FA) scalars. Eur Radiol 16:1651–1658
Funding
No funding was received.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Conflict of interest
The authors declare that they have no conflict of interest.
Ethics approval
All procedures performed in studies involving human participants were in accordance with the ethical standards of the institutional and/or national research committee and with the 1964 Helsinki Declaration and its later amendments or comparable ethical standards. As this is a retrospective study, formal consent is not required.
Informed consent
The requirement for informed consent was waived due to retrospective nature of the study.
Additional information
Publisher’s note
Springer Nature remains neutral with regard to jurisdictional claims in published maps and institutional affiliations.
Rights and permissions
About this article
Cite this article
Jeon, T.Y., Poliakov, A.V., Friedman, S.D. et al. Structural MRI and tract-based spatial statistical analysis of diffusion tensor imaging in children with hemimegalencephaly. Neuroradiology 62, 1467–1474 (2020). https://doi.org/10.1007/s00234-020-02491-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-020-02491-z