Abstract
Purpose
This paper aims to analyze the contribution of mean diffusivity (MD), axial diffusivity (AD), and radial diffusivity (RD) in the detection of microstructural abnormalities in amyotrophic lateral sclerosis (ALS) and to evaluate the degree of agreement between structural and functional changes through concomitant diffusion tensor imaging (DTI), transcranial magnetic stimulation (TMS), and clinical assessment.
Methods
Fourteen patients with ALS and 11 healthy, age- and gender-matched controls were included. All participants underwent magnetic resonance imaging including DTI. TMS was additionally performed in ALS patients. Differences in the distribution of DTI-derived measures were assessed using tract-based spatial statistical (TBSS) and volume of interest (VOI) analyses. Correlations between clinical, imaging, and neurophysiological findings were also assessed through TBSS.
Results
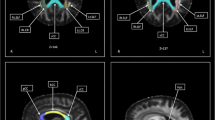
ALS patients showed a significant increase in AD and MD involving the corticospinal tract (CST) and the pre-frontal white matter in the right posterior limb of the internal capsule (p < 0.05) when compared to the control group using TBSS, confirmed by VOI analyses. VOI analyses also showed increased AD in the corpus callosum (p < 0.05) in ALS patients. Fractional anisotropy (FA) in the right CST correlated significantly with upper motor neuron (UMN) score (r = − 0.79, p < 0.05), and right abductor digiti minimi central motor conduction time was highly correlated with RD in the left posterior internal capsule (r = − 0.81, p < 0.05). No other significant correlation was found.
Conclusion
MD, AD, and RD, besides FA, are able to further detect and characterize neurodegeneration in ALS. Furthermore, TMS and DTI appear to have a role as complementary diagnostic biomarkers of UMN dysfunction.

Similar content being viewed by others
Abbreviations
- AD:
-
Axial diffusivity
- ADM:
-
Abductor digiti minimi
- AH:
-
Abductor hallucis
- ALS:
-
Amyotrophic lateral sclerosis
- ALSFRS-R:
-
Amyotrophic lateral sclerosis functional rate scale revisited
- CMAP:
-
Compound motor action potentials
- CCMCT:
-
Central motor conduction time
- CSP:
-
Cortical silent period
- CST:
-
Corticospinal tract
- DTI:
-
Diffusion tensor imaging
- FA:
-
Fractional anisotropy
- LMN:
-
Lower motor neuron
- MD:
-
Mean diffusivity
- MEP:
-
Motor evoked potential
- MRI:
-
Magnetic resonance imaging
- MT:
-
Motor threshold
- RD:
-
Radial diffusivity
- TBSS:
-
Tract-based spatial statistical
- TMS:
-
Transcranial magnetic stimulation
- UMN:
-
Upper motor neuron
- VOI:
-
Volume of interest
References
Turner MR, Hardiman O, Benatar M, Brooks BR, Chio A, de Carvalho M, Ince PG, Lin C, Miller RG, Mitsumoto H, Nicholson G, Ravits J, Shaw PJ, Swash M, Talbot K, Traynor BJ, Van den Berg LH, Veldink JH, Vucic S, Kiernan MC (2013) Controversies and priorities in amyotrophic lateral sclerosis. Lancet Neurol 12:310–312. https://doi.org/10.1016/S1474-4422(13)70036-X
de Carvalho M, Dengler R, Eisen A, England JD, Kaji R, Kimura J, Mills K, Mitsumoto H, Nodera H, Shefner J, Swash M (2008) Electrodiagnostic criteria for diagnosis of ALS. Clin Neurophysiol 119:497–403. https://doi.org/10.1016/j.clinph.2007.09.143
Chiò A, Pagani M, Agosta F, Calvo A, Cistaro A, Filippi M (2014) Neuroimaging in amyotrophic lateral sclerosis: insights into structural and functional changes. Lancet Neurol 13:1228–1240. https://doi.org/10.1016/S1474-4422(14)70167-X
Foerster BR, Welsh RC, Feldman EL (2013) 25 years of neuroimaging in amyotrophic lateral sclerosis. Nat Rev Neurol 9:513–524. https://doi.org/10.1038/nrneurol.2013.153
Chenevert TL, Brunberg JA, Pipe JG (1990) Anisotropic diffusion in human white matter: demonstration with MR techniques in vivo. Radiology 177:401–405
Agosta F, Pagani E, Petrolini M, Caputo D, Perini M, Prelle A, Salvi F, Filippi M (2010) Assessment of white matter tract damage in patients with amyotrophic lateral sclerosis: a diffusion tensor MR imaging tractography study. AJNR Am J Neuroradiol 31:1457–1461. https://doi.org/10.3174/ajnr.A2105
Metwalli NS, Benatar M, Nair G, Usher S, Hu X, Carew JD (2010) Utility of axial and radial diffusivity from diffusion tensor MRI as markers of neurodegeneration in amyotrophic lateral sclerosis. Brain Res 1348:156–164. https://doi.org/10.1016/j.brainres.2010.05.067
Canu E, Agosta F, Riva N, Sala S, Prelle A, Caputo D, Perini M, Comi G, Filippi M (2011) The topography of brain microstructural damage in amyotrophic lateral sclerosis assessed using diffusion tensor MR imaging. AJNR Am J Neuroradiol 32(7):1307–1314. https://doi.org/10.3174/ajnr.A2469
Cirillo M, Esposito F, Tedeschi G, Caiazzo G, Sagnelli A, Piccirillo G, Conforti R, Tortora F, Monsurrò MR, Cirillo S, Trojsi F (2012) Widespread microstructural white matter involvement in amyotrophic lateral sclerosis: a whole-brain DTI study. Am J Neuroradiol 33:1102–8.18. https://doi.org/10.3174/ajnr.A2918
Rajagopalan V, Yue GH, Pioro EP (2013) Brain white matter diffusion tensor metrics from clinical 1.5 T MRI distinguish between ALS phenotypes. J Neurol 260(10):2532–2540. https://doi.org/10.1007/s00415-013-7012-1
Song SK, Sun SW, Ramsbottom MJ, Chang C, Russell J, Cross AH (2002) Dysmyelination revealed through MRI as increased radial (but unchanged axial) diffusion of water. NeuroImage 17(3):1429–1436
Song SK, Yoshino J, Le TQ, Lin SJ, Sun SW, Cross AH, Armstrong RC (2005) Demyelination increases radial diffusivity in corpus callosum of mouse brain. NeuroImage 26(1):132–140
Janve VA, Zu Z, Yao SY, Li K, Zhang FL, Wilson KJ, Ou X, Does MD, Subramaniam S, Gochberg DF (2013) The radial diffusivity and magnetization transfer pool size ratio are sensitive markers for demyelination in a rat model of type III multiple sclerosis (MS) lesions. NeuroImage 74:298–305. https://doi.org/10.1016/j.neuroimage.2013.02.034
Klistorner A, Wang C, Fofanova V, Barnett MH, Yiannikas C, Parratt J, You Y, Graham SL (2016) Diffusivity in multiple sclerosis lesions: at the cutting edge? Neuroimage Clin 12:219–226. https://doi.org/10.1016/j.nicl.2016.07.003
Claus D, Brunholzl C, Kerling FP, Henschel S (1995) Transcranial magnetic stimulation as a diagnostic and prognostic test in amyotrophic lateral sclerosis. J Neurol Sci 129:30–34
Miscio G, Pisano F, Mora G, Mazzini L (1999) Motor neuron disease: usefulness of transcranial magnetic stimulation in improving diagnosis. Clin Neurophysiol 110:975–981
Triggs WJ, Menkes D, Onorato J, Yan RS, Young MS, Newell K, Sander HW, Soto O, Chiappa KH, Cros D (1999) Transcranial magnetic stimulation identifies upper motor neuron involvement in motor neuron disease. Neurology 53:605–611
Rosler KM, Truffert A, Hess CW, Magistris MR (2000) Quantification of upper motor neuron loss in amyotrophic lateral sclerosis. Clin Neurophysiol 111:2208–2218
Ellis CM, Simmons A, Jones DK, Bland J, Dawson JM, Horsfield MA, Williams SC, Leigh PN (1999) Diffusion tensor MRI assesses corticospinal tract damage in ALS. Neurology 53:1051–1058
Sach M, Winkler G, Glauche V, Liepert J, Heimbach B, Koch MA, Buchel C, Weiller C (2004) Diffusion tensor MRI of early upper motor neuron involvement in amyotrophic lateral sclerosis. Brain 127:340–350
Iwata NK, Aoki S, Okabe S, Arai N, Terao Y, Kwak S, Abe O, Kanazawa I, Tsuji S, Ugawa Y (2008) Evaluation of corticospinal tracts in ALS with diffusion tensor MRI and brainstem stimulation. Neurology 70:528–532. https://doi.org/10.1212/01.wnl.0000299186.72374.19
Furtula J, Johnsen B, Frandsen J, Rodell A, Christensen PB, Pugdahl K, Fuglsang-Frederiksen A (2013) Upper motor neuron involvement in amyotrophic lateral sclerosis evaluated by triple stimulation technique and diffusion tensor MRI. J Neurol 260:1535–1544. https://doi.org/10.1007/s00415-012-6824-8
Grapperon AM, Verschueren A, Duclos Y, Confort-Gouny S, Soulier E, Loundou AD, Guye M, Cozzone PJ, Pouget J, Ranjeva JP, Attarian S (2014) Association between structural and functional corticospinal involvement in amyotrophic lateral sclerosis assessed by diffusion tensor MRI and triple stimulation technique. Muscle Nerve 49(4):551–557. https://doi.org/10.1002/mus.23957
Bae JS, Ferguson M, Tan R, Mioshi E, Simon N, Burrell J, Vucic S, Hodges JR, Kiernan MC, Hornberger M (2000) Dissociation of structural and functional integrities of the motor system in amyotrophic lateral sclerosis and behavioral variant of frontotemporal dementia. J Clin Neurol 12(2):209–217. https://doi.org/10.3988/jcn2016.12.2.209
Brooks BR, Miller RG, Swash M, Munsat TL, World Federation of Neurology Research Group on Motor Neuron Diseases (2000) El Escorial revisited: revised criteria for the diagnosis of amyotrophic lateral sclerosis. Amyotroph Lateral Scler Other Motor Neuron Disord 1:293–299
Cedarbaum JM, Stambler N, Malta E, Fuller C, Hilt D, Thurmond B, Nakanishi A (1999) The ALSFRS-R: a revised ALS functional rating scale that incorporates assessments of respiratory function. BDNF ALS study group (phase III). J Neurol Sci 169:13–21
Bohannon RW, Smith MB (1987) Interrater reliability of a modified Ashworth scale of muscle spasticity. Phys Ther 67:206–207
Smith SM, Jenkinson M, Johansen-Berg H, Rueckert D, Nichols TE, Mackay CE, Watkins KE, Ciccarelli O, Cader MZ, Matthews PM, Behrens TE (2006) Tract-based spatial statistics: voxelwise analysis of multissubject diffusion data. NeuroImage 31:1487–1505
de Carvalho M, Swash M (2000) Nerve conduction studies in ALS. Muscle Nerve 23:344–352
Rossini PM, Barker AT, Berardelli A, Caramia MD, Caruso G, Cracco RQ, Dimitrijević MR, Hallett M, Katayama Y, Lücking CH, Maertens de Noordhout AL, Marsden CD, Murray NMF, Rothwell JC, Swash M, Tomberg C (1994) Noninvasive electrical and magnetic stimulation of the brain, spinal cord and roots: basic principles and procedures for routine clinical application. Report of an IFCN committee. Electroencephalogr Clin Neurophysiol 91:79–92
de Carvalho M, Turkman A, Swash M (2003) Motor responses evoked by transcranial magnetic stimulation and peripheral nerve stimulation in the ulnar innervation in amyotrophic lateral sclerosis: the effect of upper and lower motor neuron lesion. J Neurol Sci 210:83–90
de Carvalho M, Swash M (2010) Sensitivity of electrophysiological tests for upper and lower motor neuron dysfunction in ALS: a six-month longitudinal study. Muscle Nerve 41:208–211. https://doi.org/10.1002/mus.21495
Cerqueira V, de Mendonca A, Minez A, Dias AR, de Carvalho M (2006) Does caffeine modify cortical motor excitability? Neurophysiol Clin 36:219–226
Sage CA, Peeters RR, Görner A, Robberecht W, Sunaert S (2007) Quantitative diffusion tensor imaging in amyotrophic lateral sclerosis. NeuroImage 34(2):486–499
Swash M, Scholtz CL, Vowles G, Ingram DA (1988) Selective and asymmetric vulnerability of corticospinal and spinocerebellar tracts in motor neuron disease. J Neurol Neurosurg Psychiatry 51(6):785–789
Turner MR, Wicks P, Brownstein CA, Massagli MP, Toronjo M, Talbot K, Al-Chalabi A (2011) Concordance between site of onset and limb dominance in amyotrophic lateral sclerosis. J Neurol Neurosurg Psychiatry 82(8):853–854. https://doi.org/10.1136/jnnp.2010.208413
Devine MS, Kiernan MC, Heggie S, McCombe PA, Henderson RD (2014) Study of motor asymmetry in ALS indicates an effect of limb dominance on onset and spread of weakness, and an important role for upper motor neurons. Amyotroph Lateral Scler Frontotemporal Degener 15(7–8):481–487. https://doi.org/10.3109/21678421.2014.906617
de Carvalho M, Lopes A, Scotto M, Swash M (2001) Reproducibility of neurophysiological and myometric measurement in the ulnar nerve—abductor digiti minimi system. Muscle Nerve 24(10):1391–1395
Filippini N, Douaud G, Mackay CE, Knight S, Talbot K, Turner MR (2010) Corpus callosum involvement is a consistent feature of amyotrophic lateral sclerosis. Neurology 75(18):1645–1652. https://doi.org/10.1212/WNL.0b013e3181fb84d1
Hughes JT (1982) Pathology of amyotrophic lateral sclerosis. Adv Neurol 36:61–74
Graves MC, Fiala M, Dinglasan LA, Liu NQ, Sayre J, Chiappelli F, van Kooten C, Vinters HV (2004) Inflammation in amyotrophic lateral sclerosis spinal cord and brain is mediated by activated macrophages, mast cells and T cells. Amyotroph Lateral Scler Other Motor Neuron Disord 5(4):213–219
Menke RA, Agosta F, Grosskreutz J, Filippi M, Turner MR (2017) Neuroimaging endpoints in amyotrophic lateral sclerosis. Neurotherapeutics 14:11–23. https://doi.org/10.1007/s13311-016-0484-9
Foerster BR, Dwamena BA, Petrou M, Carlos RC, Callaghan BC, Pomper MG (2012) Diagnostic accuracy using diffusion tensor imaging in the diagnosis of ALS: a meta-analysis. Acad Radiol 19:1075–1086. https://doi.org/10.1016/j.acra.2012.04.012
Bede P, Hardiman O (2014) Lessons of ALS imaging: pitfalls and future directions a critical review. Neuroimage Clin 4:436–443. https://doi.org/10.1016/j.nicl.2014.02.011
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
No funding was received for this study.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
All procedures performed in studies involving human participants were in accordance with ethical standards of the institutional and/or national research committee and with the 1964 Helsinki declaration and its later amendments or comparable ethical standards.
Informed consent
Informed consent was obtained from all individual participants included in the study.
Rights and permissions
About this article
Cite this article
Geraldo, A.F., Pereira, J., Nunes, P. et al. Beyond fractional anisotropy in amyotrophic lateral sclerosis: the value of mean, axial, and radial diffusivity and its correlation with electrophysiological conductivity changes. Neuroradiology 60, 505–515 (2018). https://doi.org/10.1007/s00234-018-2012-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-018-2012-6