Abstract
Purpose
The aim of this study is to evaluate the MR imaging behavior of ferrous (Fe2+) and ferric (Fe3+) iron ions in order to develop a noninvasive technique to quantitatively differentiate between both forms of iron.
Methods
MRI was performed at 3 T in a phantom consisting of 21 samples with different concentrations of ferrous and ferric chloride solutions (between 0 and 10 mmol/L). A multi-echo spoiled gradient-echo pulse sequence with eight echoes was used for both T 2* and quantitative susceptibility measurements. The transverse relaxation rate, R 2* = 1/T 2*, was determined by nonlinear exponential fitting based on the mean signals in each sample. The susceptibilities, χ, of the samples were calculated after phase unwrapping and background field removal by fitting the spatial convolution of a unit dipole response to the measured internal field map. Relaxation rate changes, ΔR 2*(c Fe), and susceptibility changes, Δχ(c Fe), their linear slopes, as well as the ratios ΔR 2*(c Fe) / Δχ(c Fe) were determined for all concentrations.
Results
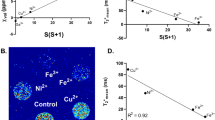
The linear slopes of the relaxation rate were (12.5 ± 0.4) s−1/(mmol/L) for Fe3+ and (0.77 ± 0.09) s−1/(mmol/L) for Fe2+ (significantly different, z test P < 0.0001). The linear slopes of the susceptibility were (0.088 ± 0.003) ppm/(mmol/L) for Fe3+ and (0.079 ± 0.006) ppm/(mmol/L) for Fe2+. The individual ratios ΔR 2*/Δχ were greater than 40 s−1/ppm for all samples with ferric solution and lower than 20 s−1/ppm for all but one of the samples with ferrous solution.
Conclusion
Ferrous and ferric iron ions show significantly different relaxation behaviors in MRI but similar susceptibility patterns. These properties can be used to differentiate ferrous and ferric samples.





Similar content being viewed by others
References
Singh N, Haldar S, Tripathi AK, Horback K, Wong J, Sharma D, Beserra A, Suda S, Anbalagan C, Dev S et al (2014) Brain iron homeostasis: from molecular mechanisms to clinical significance and therapeutic opportunities. Antioxid Redox Signal 20:1324–1363. doi:10.1089/ars.2012.4931
He N, Ling H, Ding B, Huang J, Zhang Y, Zhang Z, Liu C, Chen K, Yan F (2015) Region-specific disturbed iron distribution in early idiopathic Parkinson’s disease measured by quantitative susceptibility mapping. Hum Brain Mapp 36:4407–4420. doi:10.1002/hbm.22928
Schweitzer AD, Liu T, Gupta A, Zheng K, Seedial S, Shtilbans A, Shahbazi M, Lange D, Wang Y, Tsiouris AJ (2015) Quantitative susceptibility mapping of the motor cortex in amyotrophic lateral sclerosis and primary lateral sclerosis. AJR Am J Roentgenol 204:1086–1092. doi:10.2214/AJR.14.13459
Dominguez JF, Ng AC, Poudel G, Stout JC, Churchyard A, Chua P, Egan GF, Georgiou-Karistianis N (2016) Iron accumulation in the basal ganglia in Huntington’s disease: cross-sectional data from the IMAGE-HD study. J Neurol Neurosurg Psychiatry 87:545–549. doi:10.1136/jnnp-2014-310183
Belaidi AA, Bush AI (2016) Iron neurochemistry in Alzheimer’s disease and Parkinson’s disease: targets for therapeutics. J Neurochem 139(Suppl 1):179–197. doi:10.1111/jnc.13425
Schneider SA, Bhatia KP (2013) Excess iron harms the brain: the syndromes of neurodegeneration with brain iron accumulation (NBIA). J Neural Transm (Vienna) 120:695–703. doi:10.1007/s00702-012-0922-8
Popescu BF, George MJ, Bergmann U, Garachtchenko AV, Kelly ME, McCrea RP, Luning K, Devon RM, George GN, Hanson AD et al (2009) Mapping metals in Parkinson’s and normal brain using rapid-scanning x-ray fluorescence. Phys Med Biol 54:651–663. doi:10.1088/0031-9155/54/3/012
Sian-Hulsmann J, Mandel S, Youdim MB, Riederer P (2011) The relevance of iron in the pathogenesis of Parkinson’s disease. J Neurochem 118:939–957. doi:10.1111/j.1471-4159.2010.07132.x
Kostka M, Hogen T, Danzer KM, Levin J, Habeck M, Wirth A, Wagner R, Glabe CG, Finger S, Heinzelmann U et al (2008) Single particle characterization of iron-induced pore-forming alpha-synuclein oligomers. J Biol Chem 283:10992–11003. doi:10.1074/jbc.M709634200
Hillmer AS, Putcha P, Levin J, Hogen T, Hyman BT, Kretzschmar H, McLean PJ, Giese A (2010) Converse modulation of toxic alpha-synuclein oligomers in living cells by N’-benzylidene-benzohydrazide derivates and ferric iron. Biochem Biophys Res Commun 391:461–466. doi:10.1016/j.bbrc.2009.11.080
Wesemann W, Blaschke S, Solbach M, Grote C, Clement HW, Riederer P (1994) Intranigral injected iron progressively reduces striatal dopamine metabolism. J Neural Transm Park Dis Dement Sect 8:209–214
Logroscino G, Gao X, Chen H, Wing A, Ascherio A (2008) Dietary iron intake and risk of Parkinson’s disease. Am J Epidemiol 168:1381–1388. doi:10.1093/aje/kwn273
Berg D (2007) Disturbance of iron metabolism as a contributing factor to SN hyperechogenicity in Parkinson’s disease: implications for idiopathic and monogenetic forms. Neurochem Res 32:1646–1654. doi:10.1007/s11064-007-9346-5
Hallgren B, Sourander P (1958) The effect of age on the non-haemin iron in the human brain. J Neurochem 3:41–51
Zecca L, Gallorini M, Schunemann V, Trautwein AX, Gerlach M, Riederer P, Vezzoni P, Tampellini D (2001) Iron, neuromelanin and ferritin content in the substantia nigra of normal subjects at different ages: consequences for iron storage and neurodegenerative processes. J Neurochem 76:1766–1773
Sofic E, Riederer P, Heinsen H, Beckmann H, Reynolds GP, Hebenstreit G, Youdim MB (1988) Increased iron (III) and total iron content in post mortem substantia nigra of parkinsonian brain. J Neural Transm 74:199–205
Haacke EM, Cheng NY, House MJ, Liu Q, Neelavalli J, Ogg RJ, Khan A, Ayaz M, Kirsch W, Obenaus A (2005) Imaging iron stores in the brain using magnetic resonance imaging. Magn Reson Imaging 23:1–25. doi:10.1016/j.mri.2004.10.001
Langkammer C, Krebs N, Goessler W, Scheurer E, Ebner F, Yen K, Fazekas F, Ropele S (2010) Quantitative MR imaging of brain iron: a postmortem validation study. Radiology 257:455–462. doi:10.1148/radiol.10100495
Langkammer C, Krebs N, Goessler W, Scheurer E, Yen K, Fazekas F, Ropele S (2012) Susceptibility induced gray-white matter MRI contrast in the human brain. NeuroImage 59:1413–1419. doi:10.1016/j.neuroimage.2011.08.045
Uddin MN, Lebel RM, Wilman AH (2016) Value of transverse relaxometry difference methods for iron in human brain. Magn Reson Imaging 34:51–59. doi:10.1016/j.mri.2015.09.002
Kesavadas C, Santhosh K, Thomas B, Gupta AK, Kapilamoorthy TR, Bodhey N, Pendharker H, Patro S (2009) Signal changes in cortical laminar necrosis—evidence from susceptibility-weighted magnetic resonance imaging. Neuroradiology 51:293–298. doi:10.1007/s00234-009-0497-8
Rumzan R, Wang JJ, Zeng C, Chen X, Li Y, Luo T, Lv F, Wang ZP, Hou H, Huang F (2013) Iron deposition in the precentral grey matter in patients with multiple sclerosis: a quantitative study using susceptibility-weighted imaging. Eur J Radiol 82:e95–e99. doi:10.1016/j.ejrad.2012.09.006
Xu X, Wang Q, Zhong J, Zhang M (2015) Iron deposition influences the measurement of water diffusion tensor in the human brain: a combined analysis of diffusion and iron-induced phase changes. Neuroradiology 57:1169–1178. doi:10.1007/s00234-015-1579-4
Liu M, Liu S, Ghassaban K, Zheng W, Dicicco D, Miao Y, Habib C, Jazmati T, Haacke EM (2016) Assessing global and regional iron content in deep gray matter as a function of age using susceptibility mapping. J Magn Reson Imaging 44:59–71. doi:10.1002/jmri.25130
Gore JC, Kang YS, Schulz RJ (1984) Measurement of radiation dose distributions by nuclear magnetic resonance (NMR) imaging. Phys Med Biol 29:1189–1197
Tokuhiro T, Appleby A, Leghrouz A, Metcalf R, Tokarz R (1996) Proton spin-lattice relaxation of water molecules in ferrous-ferric/agarose gel system. J Chem Phys 105:3761–3769. doi:10.1063/1.472196
Fricke H, Morse S (1927) The chemical action of roentgen rays on dilute ferrosulphate solutions as a measure of dose. Am J Roentgenol Radium Ther 18:430–432
Surry KJ, Austin HJ, Fenster A, Peters TM (2004) Poly(vinyl alcohol) cryogel phantoms for use in ultrasound and MR imaging. Phys Med Biol 49:5529–5546
Reinertsen I, Collins DL (2006) A realistic phantom for brain-shift simulations. Med Phys 33:3234–3240. doi:10.1118/1.2219091
Abdul-Rahman HS, Gdeisat MA, Burton DR, Lalor MJ, Lilley F, Moore CJ (2007) Fast and robust three-dimensional best path phase unwrapping algorithm. Appl Opt 46:6623–6635. doi:10.1364/AO.46.006623
Schweser F, Deistung A, Lehr BW, Reichenbach JR (2010) Differentiation between diamagnetic and paramagnetic cerebral lesions based on magnetic susceptibility mapping. Med Phys 37:5165–5178
Schweser F, Deistung A, Lehr BW, Reichenbach JR (2011) Quantitative imaging of intrinsic magnetic tissue properties using MRI signal phase: an approach to in vivo brain iron metabolism? NeuroImage 54:2789–2807. doi:10.1016/j.neuroimage.2010.10.070
de Rochefort L, Brown R, Prince MR, Wang Y (2008) Quantitative MR susceptibility mapping using piece-wise constant regularized inversion of the magnetic field. Magn Reson Med 60:1003–1009. doi:10.1002/mrm.21710
Clogg CC, Petkova E, Haritou A (1995) Statistical methods for comparing regression coefficients between models. Am J Sociol 100:1261–1293
Fulay PP (2010) Electronic, magnetic, and optical materials. CRC Press, Boca Raton, London, New York
Acosta-Cabronero J, Cardenas-Blanco A, Betts MJ, Butryn M, Valdes-Herrera JP, Galazky I, Nestor PJ (2017) The whole-brain pattern of magnetic susceptibility perturbations in Parkinson’s disease. Brain 140:118–131. doi:10.1093/brain/aww278
van Bergen JM, Li X, Hua J, Schreiner SJ, Steininger SC, Quevenco FC, Wyss M, Gietl AF, Treyer V, Leh SE et al (2016) Colocalization of cerebral iron with amyloid beta in mild cognitive impairment. Sci Rep 6:35514. doi:10.1038/srep35514
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was partly funded by the Lüneburg Heritage and Deutsche Forschungsgesellschaft (DFG) Grant BO 1895/4-1 to KB.
Conflict of interest
The authors declare that they have no conflict of interest.
Ethical approval
This article does not contain any studies with human participants or animals performed by any of the authors. For this type of study, formal consent is not required.
Informed consent
Statement of informed consent was not applicable since the manuscript does not contain any patient data.
Rights and permissions
About this article
Cite this article
Dietrich, O., Levin, J., Ahmadi, SA. et al. MR imaging differentiation of Fe2+ and Fe3+ based on relaxation and magnetic susceptibility properties. Neuroradiology 59, 403–409 (2017). https://doi.org/10.1007/s00234-017-1813-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-017-1813-3