Abstract
Introduction
The aim of this study is to investigate perfusion characteristics of glioblastoma with an oligodendroglioma component (GBMO) compared with conventional glioblastoma (GBM) using dynamic susceptibility contrast (DSC) perfusion magnetic resonance (MR) imaging and microvessel density (MVD).
Methods
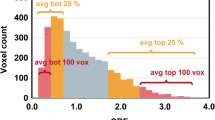
The study was approved by the institutional review board. Newly diagnosed high-grade glioma patients were enrolled (n = 72; 20 GBMs, 14 GBMOs, 19 anaplastic astrocytomas (AAs), 13 anaplastic oligodendrogliomas (AOs), and six anaplastic oligoastrocytomas (AOAs)). All participants underwent preoperative MR imaging including DSC perfusion MR imaging. Normalized cerebral blood volume (nCBV) values were analyzed using a histogram approach. Histogram parameters were subsequently compared across each tumor subtype and grade. MVD was quantified by immunohistochemistry staining and correlated with perfusion parameters. Progression-free survival (PFS) was assessed according to the tumor subtype.
Results
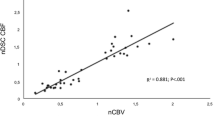
GBMO displayed significantly reduced nCBV values compared with GBM, whereas grade III tumors with oligodendroglial components (AO and AOA) exhibited significantly increased nCBV values compared with AA (p < 0.001). MVD analyses revealed the same pattern as nCBV results. In addition, a positive correlation between MVD and nCBV values was noted (r = 0.633, p < 0.001). Patients with oligodendroglial tumors exhibited significantly increased PFS compared with patients with pure astrocytomas in each grade.
Conclusion
In contrast to grade III tumors, the presence of oligodendroglial components in grade IV tumors resulted in paradoxically reduced perfusion metrics and MVD. In addition, patients with GBMO exhibited a better clinical outcome compared with patients with GBM.







Similar content being viewed by others
References
Tortosa A, Vinolas N, Villa S, Verger E, Gil JM, Brell M, Caral L et al (2003) Prognostic implication of clinical, radiologic, and pathologic features in patients with anaplastic gliomas. Cancer 97:1063–1071
Kanno H, Nishihara H, Narita T, Yamaguchi S, Kobayashi H, Tanino M, Kimura T et al (2012) Prognostic implication of histological oligodendroglial tumor component: clinicopathological analysis of 111 cases of malignant gliomas. PLoS One 7, e41669
van den Bent M, Chinot OL, Cairncross JG (2003) Recent developments in the molecular characterization and treatment of oligodendroglial tumors. Neuro Oncol 5:128–138
Ricard D, Idbaih A, Ducray F, Lahutte M, Hoang-Xuan K, Delattre JY (2012) Primary brain tumours in adults. Lancet 379:1984–1996
Cairncross G, Wang M, Shaw E, Jenkins R, Brachman D, Buckner J, Fink K et al (2013) Phase III trial of chemoradiotherapy for anaplastic oligodendroglioma: long-term results of RTOG 9402. J Clin Oncol 31:337–343
van den Bent MJ, Brandes AA, Taphoorn MJ, Kros JM, Kouwenhoven MC, Delattre JY, Bernsen HJ et al (2013) Adjuvant procarbazine, lomustine, and vincristine chemotherapy in newly diagnosed anaplastic oligodendroglioma: long-term follow-up of EORTC brain tumor group study 26951. J Clin Oncol 31:344–350
Louis DN, Ohgaki H, Wiestler OD, Cavenee WK, Burger PC, Jouvet A, Scheithauer BW et al (2007) The 2007 WHO classification of tumours of the central nervous system. Acta Neuropathol 114:97–109
Wang Y, Li S, Chen L, You G, Bao Z, Yan W, Shi Z et al (2012) Glioblastoma with an oligodendroglioma component: distinct clinical behavior, genetic alterations, and outcome. Neuro Oncol 14:518–525
Salvati M, Formichella AI, D’Elia A, Brogna C, Frati A, Giangaspero F, Delfini R et al (2009) Cerebral glioblastoma with oligodendrogliomal component: analysis of 36 cases. J Neurooncol 94:129–134
Laxton RC, Popov S, Doey L, Jury A, Bhangoo R, Gullan R, Chandler C et al (2013) Primary glioblastoma with oligodendroglial differentiation has better clinical outcome but no difference in common biological markers compared with other types of glioblastoma. Neuro Oncol 15:1635–1643
Appin CL, Gao J, Chisolm C, Torian M, Alexis D, Vincentelli C, Schniederjan MJ et al (2013) Glioblastoma with oligodendroglioma component (GBM-O): molecular genetic and clinical characteristics. Brain Pathol 23:454–461
Lev MH, Ozsunar Y, Henson JW, Rasheed AA, Barest GD, Harsh GR, Fitzek MM et al (2004) Glial tumor grading and outcome prediction using dynamic spin-echo MR susceptibility mapping compared with conventional contrast-enhanced MR: confounding effect of elevated rCBV of oligodendrogliomas [corrected]. AJNR Am J Neuroradiol 25:214–221
Cha S, Tihan T, Crawford F, Fischbein NJ, Chang S, Bollen A, Nelson SJ et al (2005) Differentiation of low-grade oligodendrogliomas from low-grade astrocytomas by using quantitative blood-volume measurements derived from dynamic susceptibility contrast-enhanced MR imaging. AJNR Am J Neuroradiol 26:266–273
Saito T, Yamasaki F, Kajiwara Y, Abe N, Akiyama Y, Kakuda T, Takeshima Y et al (2012) Role of perfusion-weighted imaging at 3T in the histopathological differentiation between astrocytic and oligodendroglial tumors. Eur J Radiol 81:1863–1869
Emblem KE, Scheie D, Due-Tonnessen P, Nedregaard B, Nome T, Hald JK, Beiske K et al (2008) Histogram analysis of MR imaging-derived cerebral blood volume maps: combined glioma grading and identification of low-grade oligodendroglial subtypes. AJNR Am J Neuroradiol 29:1664–1670
Wen PY, Macdonald DR, Reardon DA, Cloughesy TF, Sorensen AG, Galanis E, Degroot J et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28:1963–1972
Ostergaard L, Weisskoff RM, Chesler DA, Gyldensted C, Rosen BR (1996) High resolution measurement of cerebral blood flow using intravascular tracer bolus passages. Part I: Mathematical approach and statistical analysis. Magn Reson Med 36:715–725
Boxerman JL, Schmainda KM, Weisskoff RM (2006) Relative cerebral blood volume maps corrected for contrast agent extravasation significantly correlate with glioma tumor grade, whereas uncorrected maps do not. AJNR Am J Neuroradiol 27:859–867
Bjornerud A, Sorensen AG, Mouridsen K, Emblem KE (2011) T1- and T2*-dominant extravasation correction in DSC-MRI: part I—theoretical considerations and implications for assessment of tumor hemodynamic properties. J Cereb Blood Flow Metab 31:2041–2053
Tozer DJ, Jager HR, Danchaivijitr N, Benton CE, Tofts PS, Rees JH, Waldman AD (2007) Apparent diffusion coefficient histograms may predict low-grade glioma subtype. NMR Biomed 20:49–57
Kang Y, Choi SH, Kim YJ, Kim KG, Sohn CH, Kim JH, Yun TJ et al (2011) Gliomas: Histogram analysis of apparent diffusion coefficient maps with standard- or high-b-value diffusion-weighted MR imaging—correlation with tumor grade. Radiology 261:882–890
Scheie D, Andresen PA, Cvancarova M, Bo AS, Helseth E, Skullerud K, Beiske K (2006) Fluorescence in situ hybridization (FISH) on touch preparations: a reliable method for detecting loss of heterozygosity at 1p and 19q in oligodendroglial tumors. Am J Surg Pathol 30:828–837
Law M, Young RJ, Babb JS, Peccerelli N, Chheang S, Gruber ML, Miller DC et al (2008) Gliomas: predicting time to progression or survival with cerebral blood volume measurements at dynamic susceptibility-weighted contrast-enhanced perfusion MR imaging. Radiology 247:490–498
Hirai T, Murakami R, Nakamura H, Kitajima M, Fukuoka H, Sasao A, Akter M et al (2008) Prognostic value of perfusion MR imaging of high-grade astrocytomas: long-term follow-up study. AJNR Am J Neuroradiol 29:1505–1510
Jain R, Poisson L, Narang J, Gutman D, Scarpace L, Hwang SN, Holder C et al (2013) Genomic mapping and survival prediction in glioblastoma: molecular subclassification strengthened by hemodynamic imaging biomarkers. Radiology 267:212–220
Cairncross JG, Ueki K, Zlatescu MC, Lisle DK, Finkelstein DM, Hammond RR, Silver JS et al (1998) Specific genetic predictors of chemotherapeutic response and survival in patients with anaplastic oligodendrogliomas. J Natl Cancer Inst 90:1473–1479
Ren X, Cui X, Lin S, Wang J, Jiang Z, Sui D, Li J et al (2012) Co-deletion of chromosome 1p/19q and IDH1/2 mutation in glioma subsets of brain tumors in Chinese patients. PLoS One 7, e32764
Eoli M, Bissola L, Bruzzone MG, Pollo B, Maccagnano C, De Simone T, Valletta L et al (2006) Reclassification of oligoastrocytomas by loss of heterozygosity studies. Int J Cancer 119:84–90
Mizoguchi M, Yoshimoto K, Ma X, Guan Y, Hata N, Amano T, Nakamizo A et al (2012) Molecular characteristics of glioblastoma with 1p/19q co-deletion. Brain Tumor Pathol 29:148–153
He J, Mokhtari K, Sanson M, Marie Y, Kujas M, Huguet S, Leuraud P et al (2001) Glioblastomas with an oligodendroglial component: a pathological and molecular study. J Neuropathol Exp Neurol 60:863–871
Jenkinson MD, Smith TS, Joyce KA, Fildes D, Broome J, du Plessis DG, Haylock B et al (2006) Cerebral blood volume, genotype and chemosensitivity in oligodendroglial tumours. Neuroradiology 48:703–713
Yan H, Parsons DW, Jin G, McLendon R, Rasheed BA, Yuan W, Kos I et al (2009) IDH1 and IDH2 mutations in gliomas. N Engl J Med 360:765–773
Ha SY, Kang SY, Do IG, Suh YL (2013) Glioblastoma with oligodendroglial component represents a subgroup of glioblastoma with high prevalence of IDH1 mutation and association with younger age. J Neurooncol 112:439–448
Labussiere M, Sanson M, Idbaih A, Delattre JY (2010) IDH1 gene mutations: a new paradigm in glioma prognosis and therapy? Oncologist 15:196–199
Sadeghi N, D’Haene N, Decaestecker C, Levivier M, Metens T, Maris C, Wikler D et al (2008) Apparent diffusion coefficient and cerebral blood volume in brain gliomas: relation to tumor cell density and tumor microvessel density based on stereotactic biopsies. AJNR Am J Neuroradiol 29:476–482
Aronen HJ, Gazit IE, Louis DN, Buchbinder BR, Pardo FS, Weisskoff RM, Harsh GR et al (1994) Cerebral blood volume maps of gliomas: comparison with tumor grade and histologic findings. Radiology 191:41–51
Cha S, Johnson G, Wadghiri YZ, Jin O, Babb J, Zagzag D, Turnbull DH (2003) Dynamic, contrast-enhanced perfusion MRI in mouse gliomas: correlation with histopathology. Magn Reson Med 49:848–855
Jain R, Gutierrez J, Narang J, Scarpace L, Schultz LR, Lemke N, Patel SC et al (2011) In vivo correlation of tumor blood volume and permeability with histologic and molecular angiogenic markers in gliomas. AJNR Am J Neuroradiol 32:388–394
Acknowledgments
This study was supported by a grant from the Korea Healthcare Technology R&D Projects, Ministry for Health, Welfare and Family Affairs (HI13C0015) and by the Research Center Program of IBS (Institute for Basic Science) in Korea.
Ethical Standards and Patient Consent
We declare that all human and animal studies have been approved by our Institutional Review Board and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that due to the retrospective nature of the study, informed consent was waived.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sunwoo, L., Choi, S.H., Yoo, RE. et al. Paradoxical perfusion metrics of high-grade gliomas with an oligodendroglioma component: quantitative analysis of dynamic susceptibility contrast perfusion MR imaging. Neuroradiology 57, 1111–1120 (2015). https://doi.org/10.1007/s00234-015-1569-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-015-1569-6