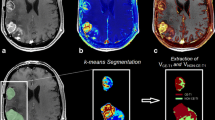
Abstract
Introduction
Treatment with the humanized anti-vascular endothelial growth factor (VEGF) antibody bevacizumab in glioblastoma patients suppresses contrast enhancement via the reduction of vascular permeability, which does not necessarily indicate real reduction of tumor cell mass. Therefore, other imaging criteria are needed to recognize tumor growth under bevacizumab more reliably. It is still unknown, whether quantitative T1 mapping is useful to monitor the effects of anti-angiogenic therapy or to indicate a tumor progression earlier and more reliable compared to conventional magnetic resonance imaging (MRI) sequences. This raised the question whether quantitative T1 mapping is more suitable to monitor treatment effects of bevacizumab.
Methods
Conventional and quantitative MRI was performed on six consecutive patients with recurrent glioblastoma before treatment with bevacizumab and every 8 weeks thereafter until further tumor progression. Quantitative T1 maps before and after intravenous application of contrast agent and quantitative T2 maps were performed to calculate serial differential maps and subtraction maps from one time point, subtracting contrast-enhanced T1 maps from non-contrast T1 maps.
Results
In five illustrative cases, tumor progression was documented earlier in differential T1 relaxation time (DiffT1) and T2 relaxation time (DiffT2) maps before changes in the conventional MRI studies were obvious. Four patients showed previous prolongation of T1 relaxation time in the DiffT1 maps, suggesting tumor progression, and subtraction maps revealed faint contrast enhancement matching with the areas of T1 prolongation.
Conclusion
Our results emphasize that quantitative relaxation time mapping could be a promising method for tumor monitoring in glioblastoma patients under anti-angiogenic therapy. Quantitative T1 mapping seems to detect enhancing tumor earlier than conventional contrast-enhanced T1-weighted images.




Similar content being viewed by others
References
Stupp R, Mason WP, van den Bent MJ et al (2005) Radiotherapy plus concomitant and adjuvant temozolomide for glioblastoma. N Engl J Med 352(10):987–996
Wong ET, Hess KR, Gleason MJ et al (1999) Outcomes and prognostic factors in recurrent glioma patients enrolled onto phase II clinical trials. J Clin Oncol 17:2572–2578
Saitta L, Heese O, Förster AF et al (2011) Signal intensity in T2′ magnetic resonance imaging is related to brain glioma grade. Eur Radiol 21(5):1068–1076
Wick W, Puduvalli VK, Chamberlain MC et al (2010) Phase III study of enzastaurin compared with lomustine in the treatment of recurrent intracranial glioblastoma. J Clin Oncol 28(7):1168–1174
Vredenburgh JJ, Desjardins A, Herndon JE et al (2007) Bevacizumab plus irinotecan in recurrent glioblastoma multiforme. J Clin Oncol 25(30):4722–4729
Friedman HS, Prados MD, Wen PY et al (2009) Bevacizumab alone and in combination with irinotecan in recurrent glioblastoma. J Clin Oncol 27(28):4733–4740
Stark-Vance V (2005) Bevacizumab and CPT-11 in the treatment of relapsed malignant glioma [abstract]. Proceedings of the World Federation of Neuro-Oncology Meeting. Neuro-Oncol 7:369
Wagner SA, Desjardins A, Reardon DA et al (2008) Update on survival from the original phase II trial of bevacizumab and irinotecan in recurrent malignant gliomas [abstract]. J Clin Oncol 26:2021
Claes A, Gambarota G, Hamans B et al (2008) Magnetic resonance imaging-based detection of glial brain tumors in mice after antiangiogenic treatment. Int J Cancer 22:1981–1986
Norden AD, Young GS, Setayesh K et al (2008) Bevacizumab for recurrent malignant gliomas: efficacy, toxicity, and patterns of recurrence. Neurology 70:779–787
Macdonald D, Cascino T, Schold SJ et al (1990) Response criteria for phase II studies of supratentorial malignant glioma. J Clin Oncol 8(7):1277–1280
Casanovas O, Hicklin DJ, Bergers G et al (2005) Drug resistance by evasion of antiangiogenic targeting of VEGF signaling in late-stage pancreatic islet tumors. Cancer Cell 8(4):299–309
da Cruz LC H Jr, Rodriguez I, Domingues RC et al (2011) Pseudoprogression and pseudoresponse: imaging challenges in the assessment of posttreatment glioma. Am J Neuroradiol 32(11):1978–1985
Brandsma D, van den Bent MJ (2009) Pseudoprogression and pseudoresponse in the treatment of gliomas. Curr Opin Neurol 22(6):633–638, Review
Wen PY, Macdonald DR, Reardon DA et al (2010) Updated response assessment criteria for high-grade gliomas: response assessment in neuro-oncology working group. J Clin Oncol 28(11):1963–1972
Pope WB, Hessel C (2011) Response assessment in neuro-oncology criteria: implementation challenges in multicenter neuro-oncology trials. AJNR Am J Neuroradiol 32(5):794–797
Hattingen E, Jurcoane A, Daneshvar K et al (2013) Quantitative T2 mapping of recurrent glioblastoma under bevacizumab improves monitoring for non-enhancing tumor progression and predicts overall survival. Neurooncol 15(10):1395–404
Rieger J, Bähr O, Muller K et al (2010) Bevacizumab-induced diffusion-restricted lesions in malignant glioma patients. Neurooncol 99(1):49–56
Araki T, Inouye T, Suzuki H et al (1984) Magnetic resonance imaging of brain tumors: measurement of T1. Work in progress. Radiol 150(1):95–98
Englund E, Brun A, Larsson EM et al (1986) Tumours of the central nervous system. Proton magnetic resonance relaxation times T1 and T2 and histopathologic correlates. Acta Radiol Diagn 27:653–659
Le Bas JF, Leviel JL, Decorps M et al (1984) NMR relaxation times from serial stereotactic biopsies in human brain tumors. J Comput Assist Tomogr 8:1048–1057
Ellingson BM, Kim HJ, Woodworth DC et al (2014) Recurrent glioblastoma treated with bevacizumab: contrast-enhanced T1-weighted subtraction maps improve tumor delineation and aid prediction of survival in a multicenter clinical trial. Radiology 271(1):200–210
Preibisch C, Deichmann R (2009) T-1 mapping using spoiled FLASH-EPI hybrid sequences and varying flip angles. Magn Reson Imaging 62:240–246
Howarth C, Hutton C, Deichmann R (2006) Improvement of the image quality of T1-weighted anatomical brain scans. Neuroimaging 29:930–937
Venkatesan R, Lin WL, Haacke EM (1998) Accurate determination of spin-density and T-1 in the presence of RF-field inhomogeneities and flip-angle miscalibration. Magn Reson Med 40:592–602
Volz S, Noth U, Rotarska-Jagiela A et al (2010) A fast B1-mapping method for the correction and normalization of magnetization transfer ratio maps at 3T. Neuroimaging 49:3015–3026
Hattingen E, Jurcoane A, Bähr O et al (2011) Bevacizumab impairs oxidative energy metabolism and shows antitumoral effects in recurrent glioblastomas: a 31P/1H MRSI and quantitative magnetic resonance imaging study. Neurooncol 13(12):1349–1363
Ellingson BM, Cloughesy TF, Lai A et al (2012) Quantification of edema reduction using differential quantitative T2 (DQT2) relaxometry mapping in recurrent glioblastoma treated with bevacizumab. J Neurooncol 106(1):111–119
Jenkinson M, Beckmann CF, Behrens TE et al (2012) FSL Neuroimaging 62(2):782–790
Jurcoane A, Wagner M, Schmidt C et al (2013) Within-lesion differences in quantitative MRI parameters predict contrast enhancement in multiple sclerosis. J Magn Reson Imaging 38(6):1454–1561
Mottershead JP, Schmierer K, Clemence M et al (2003) High field MRI correlates of myelin content and axonal density in multiple sclerosis—a post-mortem study of the spinal cord. J Neurol 250:1293–1301
Seewann A, Vrenken H, van der Valk P et al (2009) Diffusely abnormal white matter in chronic multiple sclerosis: imaging and histopathologic analysis. Arch Neurol 66:601–609
van Waesberghe JH, Kamphorst W, De Groot CJ et al (1999) Axonal loss in multiple sclerosis lesions: magnetic resonance imaging insights into substrates of disability. Ann Neurol 46:747–754
Chiba Y, Sasayama T, Miyake S et al (2008) Anti-VEGF receptor antagonist (VGA1155) reduces infarction in rat permanent focal brain ischemia. Kobe J Med Sci 54(2):136–146
Deoni SC, Peters TM, Rutt BK (2004) Determination of optimal angles for variable nutation proton magnetic spin-lattice, T1, and spin-spin, T2, relaxation times measurement. Magn Reson Med 51:194–199
Deichmann R (2006) Fast structural brain imaging using an MDEFT sequence with a FLASH-EPI hybrid readout. Neuroimaging 33:1066–1071
Katz D, Taubenberger JK, Cannella B et al (1993) Correlation between magnetic resonance imaging findings and lesion development in chronic, active multiple sclerosis. Ann Neurol 34:661–669
Brück W, Bitsch A, Kolenda H et al (1997) Inflammatory central nervous system demyelination: correlation of magnetic resonance imaging findings with lesion pathology. Ann Neurol 42:783–793
Oh J, Cha S, Aiken AH et al (2005) Quantitative apparent diffusion coefficients and T2 relaxation times in characterizing contrast enhancing brain tumors and regions of peritumoral edema. J Magn Reson Imaging 21(6):701–708
Hoehn-Berlage M, Tolxdorff T, Bockhorst K et al (1992) In vivo NMRT2 relaxation of experimental brain tumors in the cat: a multiparameter tissue characterization. Magn Reson Imaging 10(6):935–947
Eis M, Els T, Hoehn-Berlage M (1995) High resolution quantitative relaxation and diffusion MRI of three different experimental brain tumors in rat. Magn Reson Med 34(6):835–844
Acknowledgments
We thank Stefanie Pellikan and Maurice Harth of the Institute of Neuroradiology and the staff and nurses of the Dr. Senckenberg Institute of Neurooncology who supported this study. Excellent technical assistance was provided by Professor Ralf Deichmann and his staff from the Brain Imaging Center Frankfurt. The Dr. Senckenberg Institute of Neurooncology is supported by the Dr. Senckenberg Foundation and the Hertie Foundation.
Ethical standards and patient consent
We declare that all human and animal studies have been approved by the local Ethics Committee at the University Hospital Frankfurt [Reference number 4/09-SIN 01/09] and have therefore been performed in accordance with the ethical standards laid down in the 1964 Declaration of Helsinki and its later amendments. We declare that all patients gave informed consent prior to inclusion in this study.
Conflict of interest
OB has served as a consultant for Roche, the European distributer of bevacizumab and has received travel grants from Roche.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(GIF 417kb)
Rights and permissions
About this article
Cite this article
Lescher, S., Jurcoane, A., Veit, A. et al. Quantitative T1 and T2 mapping in recurrent glioblastomas under bevacizumab: earlier detection of tumor progression compared to conventional MRI. Neuroradiology 57, 11–20 (2015). https://doi.org/10.1007/s00234-014-1445-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-014-1445-9