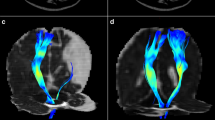
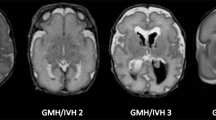
Abstract
Introduction
Our aims were to (1) assess the corticospinal tracts (CSTs) in infants with focal injury and healthy term controls using probabilistic tractography and (2) to correlate the conventional magnetic resonance imaging (MRI) and tractography findings in infants with focal injury with their later motor function.
Methods
We studied 20 infants with focal lesions and 23 controls using MRI and diffusion tensor imaging. Tract volume, fractional anisotropy (FA), apparent diffusion coefficient (ADC) values, axial diffusivity and radial diffusivity (RD) of the CSTs were determined. Asymmetry indices (AIs) were calculated by comparing ipsilateral to contralateral CSTs. Motor outcome was assessed using a standardized neurological examination.
Results
Conventional MRI was able to predict normal motor development (n = 9) or hemiplegia (n = 6). In children who developed a mild motor asymmetry (n = 5), conventional MRI predicted a hemiplegia in two and normal motor development in three infants. The AIs for tract volume, FA, ADC and RD showed a significant difference between controls and infants who developed a hemiplegia, and RD also showed a significant difference in AI between controls and infants who developed a mild asymmetry.
Conclusion
Conventional MRI was able to predict subsequent normal motor development or hemiplegia following focal injury in newborn infants. Measures of RD obtained from diffusion tractography may offer additional information for predicting a subsequent asymmetry in motor function.



Similar content being viewed by others
Abbreviations
- AD:
-
Axial diffusivity
- ADC:
-
Apparent diffusion coefficient
- AI:
-
Asymmetry index
- AIS:
-
Arterial ischemic stroke
- CP:
-
Cerebral peduncle
- CST:
-
Corticospinal tract
- DTI:
-
Diffusion tensor imaging
- DWI:
-
Diffusion weighted imaging
- FA:
-
Fractional anisotropy
- HPI:
-
Haemorrhagic parenchymal infarction
- MCA:
-
Middle cerebral artery
- MRI:
-
Magnetic resonance imaging
- PLIC:
-
Posterior limb of the internal capsule
- RD:
-
Radial diffusivity
- SI:
-
Signal intensity
- TE:
-
Echo time
- TEA:
-
Term equivalent age
- TR:
-
Repetition time
- WM:
-
White matter
References
Benders MJ, Groenendaal F, Uiterwaal CS, Nikkels PG, Bruinse HW, Nievelstein RA et al (2007) Maternal and infant characteristics associated with perinatal arterial stroke in the preterm infant. Stroke 38:1759–1765
Mercuri E, Rutherford M, Cowan F, Pennock J, Counsell S, Papadimitriou M et al (1999) Early prognostic indicators of outcome in infants with neonatal cerebral infarction: a clinical, electroencephalogram, and magnetic resonance imaging study. Pediatrics 103:39–46
deVeber GA, MacGregor D, Curtis R, Mayank S (2000) Neurologic outcome in survivors of childhood arterial ischemic stroke and sinovenous thrombosis. J Child Neurol 15:316–324
Sreenan C, Bhargava R, Robertson CM (2000) Cerebral infarction in the term newborn: clinical presentation and long-term outcome. J Pediatr 137:351–355
de Vries LS, Groenendaal F, van Haastert I, Eken P, Rademaker KJ, Meiners LC (1999) Asymmetrical myelination of the posterior limb of the internal capsule in infants with periventricular haemorrhagic infarction: an early predictor of hemiplegia. Neuropediatrics 30:314–319
Huppi PS, Maier SE, Peled S, Zientara GP, Barnes PD, Jolesz FA et al (1998) Microstructural development of human newborn cerebral white matter assessed in vivo by diffusion tensor magnetic resonance imaging. Pediatr Res 44:584–590
Neil JJ, Shiran SI, McKinstry RC, Schefft GL, Snyder AZ, Almli CR et al (1998) Normal brain in human newborns: apparent diffusion coefficient and diffusion anisotropy measured by using diffusion tensor MR imaging. Radiology 209:57–66
Seghier ML, Lazeyras F, Zimine S, Maier SE, Hanquinet S, Delavelle J et al (2004) Combination of event-related fMRI and diffusion tensor imaging in an infant with perinatal stroke. NeuroImage 21:463–472
Ward P, Counsell S, Allsop J, Cowan F, Shen Y, Edwards D et al (2006) Reduced fractional anisotropy on diffusion tensor magnetic resonance imaging after hypoxic–ischemic encephalopathy. Pediatrics 117:e619–e630
Behrens TE, Johansen-Berg H, Woolrich MW, Smith SM, Wheeler-Kingshott CA, Boulby PA et al (2003) Non-invasive mapping of connections between human thalamus and cortex using diffusion imaging. Nat Neurosci 6:750–757
Counsell SJ, Dyet LE, Larkman DJ, Nunes RG, Boardman JP, Allsop JM et al (2007) Thalamo-cortical connectivity in children born preterm mapped using probabilistic magnetic resonance tractography. NeuroImage 34:896–904
Dudink J, Mercuri E, Al-Nakib L, Govaert P, Counsell SJ, Rutherford MA et al (2009) Evolution of unilateral perinatal arterial ischemic stroke on conventional and diffusion-weighted MR imaging. Am J Neuroradiol 30:998–1004
Krishnamoorthy KS, Soman TB, Takeoka M, Schaefer PW (2000) Diffusion-weighted imaging in neonatal cerebral infarction: clinical utility and follow-up. J Child Neurol 15:592–602
Kirton A, Shroff M, Visvanathan T, deVeber G (2007) Quantified corticospinal tract diffusion restriction predicts neonatal stroke outcome. Stroke 38:974–980
de Vries LS, Van der Grond J, van Haastert IC, Groenendaal F (2005) Prediction of outcome in new-born infants with arterial ischaemic stroke using diffusion-weighted magnetic resonance imaging. Neuropediatrics 36:12–20
Gilmore JH, Lin W, Corouge I, Vetsa YS, Smith JK, Kang C et al (2007) Early postnatal development of corpus callosum and corticospinal white matter assessed with quantitative tractography. Am J Neuroradiol 28:1789–1795
Aeby A, Liu Y, De Tiege X, Denolin V, David P, Baleriaux D et al (2009) Maturation of thalamic radiations between 34 and 41 weeks’ gestation: a combined voxel-based study and probabilistic tractography with diffusion tensor imaging. Am J Neuroradiol 30:1780–1786
Smith SM, Jenkinson M, Woolrich MW, Beckmann CF, Behrens TE, Johansen-Berg H et al (2004) Advances in functional and structural MR image analysis and implementation as FSL. NeuroImage 23:S208–S219
Bassi L, Chew A, Merchant N, Ball G, Ramenghi L, Boardman J et al (2011) Diffusion tensor imaging in preterm infants with punctate white matter lesions. Pediatr Res 69:561–566
Haataja L, Mercuri E, Regev R, Cowan F, Rutherford M, Dubowitz V et al (1999) Optimality score for the neurologic examination of the infant at 12 and 18 months of age. J Pediatr 135:153–161
Choi CH, Lee JM, Koo BB, Park JS, Kim DS, Kwon JS et al (2010) Sex differences in the temporal lobe white matter and the corpus callosum: a diffusion tensor tractography study. Neuroreport 21:73–77
Mercuri E, Barnett A, Rutherford M, Guzzetta A, Haataja L, Cioni G et al (2004) Neonatal cerebral infarction and neuromotor outcome at school age. Pediatrics 113:95–100
Puig J, Pedraza S, Blasco G, Daunis IE, Prats A, Prados F et al (2010) Wallerian degeneration in the corticospinal tract evaluated by diffusion tensor imaging correlates with motor deficit 30 days after middle cerebral artery ischemic stroke. Am J Neuroradiol 31:1324–1330
Pierpaoli C, Barnett A, Pajevic S, Chen R, Penix LR, Virta A et al (2001) Water diffusion changes in Wallerian degeneration and their dependence on white matter architecture. NeuroImage 13:1174–1185
Werring DJ, Toosy AT, Clark CA, Parker GJ, Barker GJ, Miller DH et al (2000) Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J Neurol Neurosurg Psychiatry 69:269–272
Bouza H, Dubowitz LM, Rutherford M, Pennock JM (1994) Prediction of outcome in children with congenital hemiplegia: a magnetic resonance imaging study. Neuropediatrics 25:60–66
Adams E, Chau V, Poskitt KJ, Grunau RE, Synnes A, Miller SP (2010) Tractography-based quantitation of corticospinal tract development in premature newborns. J Pediatr 156:882–888
Liu Y, Baleriaux D, Kavec M, Metens T, Absil J, Denolin V et al (2010) Structural asymmetries in motor and language networks in a population of healthy preterm neonates at term equivalent age: a diffusion tensor imaging and probabilistic tractography study. NeuroImage 51:783–788
Acknowledgements
We thank the Medical Research Council (UK) and Imperial College Healthcare Comprehensive Biomedical Research Centre Funding Scheme.
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Table S1
Patient characteristics. EmCS emergency caesarean section, SVD spontaneous vaginal delivery, PROM prolonged rupture of membranes, IUGR intrauterine growth restriction, HIE hypoxic–ischaemic encephalopathy, GA gestational age, M male, F female, BMI body mass index (DOCX 15 kb)
Table S2
Diffusion characteristics of the corticospinal tracts in infants with lesions and controls. #Indicates that connectivity distributions could not be generated (DOCX 15 kb)
Figure S1A
Asymmetry index for tract volume (a), FA (b), ADC values (c), axial diffusivity (d) and radial diffusivity (e) of the corticospinal tract in newborns with ischaemic lesions and controls. AI asymmetry index. Key: normal controls (diamond), cases with normal motor outcome (square), cases who developed an asymmetry (circle), cases who developed a hemiplegia (cross) (JPEG 7 kb)
Figure S1B
(JPEG 6 kb)
Figure S1C
(JPEG 7 kb)
Figure S1D
(JPEG 6 kb)
Figure S1E
(JPEG 34 kb)
Rights and permissions
About this article
Cite this article
Roze, E., Harris, P.A., Ball, G. et al. Tractography of the corticospinal tracts in infants with focal perinatal injury: comparison with normal controls and to motor development. Neuroradiology 54, 507–516 (2012). https://doi.org/10.1007/s00234-011-0969-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-011-0969-5