Abstract
Introduction
Nuclear medicine studies in Parkinson’s disease (PD) indicate that nigrostriatal damage causes a widespread cortical hypoactivity assumed to be due to reduced excitatory thalamic outflow. However, so far, functional MRI (fMRI) studies have provided controversial data about this “functional deafferentation” phenomenon. To further clarify this issue, we assessed, with fMRI, de novo drug-naive PD patients using a relatively complex motor task under strictly controlled conditions.
Methods
Nineteen de novo PD patients with right-predominant or bilateral symptoms and 13 age-matched healthy volunteers performed continuous writing of “8” figures with the right-dominant hand using a MR-compatible device that enables identification of incorrectly performed tasks and measures the size and the frequency of the “8”s. The data were analyzed with FSL software and correlated with the clinical severity rated according to the Hoehn and Yahr (HY) staging system.
Results
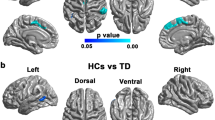
Fifteen (89%) of 19 PD patients and 12 (92%) of 13 controls correctly executed the task. PD patients showed significant hypoactivation of the left primary sensorimotor cortex (SM1) and cerebellum and no hyperactive areas as compared to controls. However, activation in SM1 and supplementary motor area bilaterally, in left supramarginal, parietal inferior, parietal superior and frontal superior gyri as well as in right parietal superior and angular gyri paralleled increasing disease severity as assessed with the HY stage.
Conclusions
In line with the “deafferentation hypothesis”, fMRI demonstrates hypoactivation of the SM1 in the early clinical stage of PD.




Similar content being viewed by others
References
Albin RL, Young AB, Penney JB (1989) The functional anatomy of basal ganglia disorders. Trends Neurosci 12:366–375
Rascol O, Sabatini U, Chollet F, Celsis P, Montastruc JL, Marc-Vergnes JP, Rascol A (1992) Supplementary and primary sensory motor area activity in Parkinson’s disease. Regional cerebral blood flow changes during finger movements and effects of apomorphine. Arch Neurol 49:144–148
Jenkins IH, Fernandez W, Playford ED, Lees AJ, Frackowiak RS, Passingham RE, Brooks DJ (1992) Impaired activation of the supplementary motor area in Parkinson’s disease is reversed when akinesia is treated with apomorphine. Ann Neurol 32:749–757
Playford ED, Jenkins IH, Passingham RE, Nutt J, Frackowiak RS, Brooks DJ (1992) Impaired mesial frontal and putamen activation in Parkinson’s disease: a positron emission tomography study. Ann Neurol 32:151–161
Jahanshahi M, Jenkins IH, Brown RG, Marsden CD, Passingham RE, Brooks DJ (1995) Self-initiated versus externally triggered movements. An investigation using measurement of regional cerebral blood flow with PET and movement-related potentials in normal and Parkinson’s disease subjects. Brain 118:913–933
Samuel M, Ceballos Baumann AO, Turjanski N, Boecker H, Gorospe A, Linazasoro G, Holmes AP, DeLong MR, Vitek JL, Thomas DG, Quinn NP, Obeso JA, Brooks DJ (1997) Pallidotomy in Parkinson’s disease increases supplementary motor area and prefrontal activation during performance of volitional movements. An H2O PET study. Brain 120:1301–1313
Eckert T, Tang C, Eidelberg D (2007) Assessing the progression of Parkinson’s disease: a metabolic network approach. Lancet Neurol 6:926–932
Teune LK, Bartels AL, De Jong BM, Willemsen AT, Eshuis SA, de Vries JJ, van Oostrom JC, Leenders KL (2010) Typical cerebral metabolic patterns in neurodegenerative brain diseases. Mov Disord 25:2395–2404
Limousin P, Greens J, Pollak P, Rothwell J, Bernabid AL, Frackowiak R (1997) Changes in cerebral activity pattern due to subthalamic nuckeus or internal pallidum stimulation in Parkinson’s disease. Ann Neurol 42:283–291
Ceballos-Baumann AO, Boecker H, Bartestenstein P, von Falkenhayn I, Riescher H, Conrad B, Moringlane JR, Alesch F (1999) A positron emission tomographic study of subthalamic nucleus stimulation in Parkinson disease: enhanced movement-related activity of motor-association cortex and decreased motor cortex resting activity. Arch Neurol 56:997–1003
Strafella AP, Dagher A, Sadikot AF (2003) Cerebral blood flow changes induced by subthalamic stimulation in Parkinson’s disease. Neurology 60:1039–1042
Buhmann C, Glauche V, Sturenburg HJ, Oechsner M, Weiller C, Buchel C (2003) Pharmacologically modulated fMRI cortical responsiveness to levodopa in drug-naive hemiparkinsonian patients. Brain 126:451–461
Tessa C, Lucetti C, Diciotti S, Baldacci F, Paoli L, Cecchi P, Giannelli M, Ginestroni A, Del Dotto P, Ceravolo R, Vignali C, Bonuccelli U, Mascalchi M (2010) Decreased and increased cortical activation coexist in de-novo Parkinson’s disease. Exp Neurol 224:299–306
Sabatini U, Boulanouar K, Fabre N, Martin F, Carel C, Colonnese C, Bozzao L, Berry I, Montastruc JL, Chollet F, Rascol O (2000) Cortical motor reorganization in akinetic patients with Parkinson’s disease: a functional MRI study. Brain 123:394–403
Haslinger B, Erhard P, Kampfe N, Boecker H, Rummeny E, Schwaiger M, Conrad B, Ceballos-Baumann AO (2001) Event-related functional magnetic resonance imaging in Parkinson’s disease before and after levodopa. Brain 124:558–570
Wu T, Hallet M (2005) A functional MRI study of automatic movements in patients with Parkinson’s disease. Brain 128:2250–2259
Eckert T, Peschel T, Heinze HJ, Rotte M (2006) Increased pre-SMA activation in early PD patients during simple self-initiated hand movements. J Neurol 253:199–207
Moraschi M, Giulietti G, Giove F, Guardati M, Garreffa G, Modugno N, Colonnese C, Maraviglia B (2010) fMRI study of motor cortex activity modulation in early Parkinson’s disease. Magn Reson Imaging 28:1152–1158
Peters S, Suchan B, Rusin J, Daum J, Kster O, Przuntek H, Muller T, Schmid G (2003) Apomorphine reduces BOLD signal in fMRI during voluntary movement in parkinsonian patients. Neuroreport 14:809–812
Dagher A, Nagano-Saito A (2007) Functional and anatomical magnetic resonance imaging in Parkinson’s disease. Mol Imaging Biol 9:234–242
Gibb WRG, Lees AJ (1988) The relevance of the Lewy body to the pathogenesis of idiopathic Parkinson’s disease. J Neurol Neurosurg Psychiatry 51:745–752
Fahn S, Elton R, the members of the Unified Parkinson's Disease Rating Scale Development Committee (1987) Unified Parkinson’s disease rating scale. In: Fahn S, Marsden CD, Calne D (eds) Recent developments in Parkinson’s disease. MacMillan, New York, pp 153–163
Hoehn MM, Yahr MD (1967) Parkinsonism: onset, progression and mortality. Neurology 17:427–442
Oldfield RC (1971) The assessment and analysis of handedness: the Edinburgh inventory. Neuropsychologia 9:97–113
Saini S, DeStefano N, Smith S, Guidi L, Amato MP, Federico A, Matthews PM (2004) Altered cerebellar functional connectivity mediates potential adaptive plasticity in patients with multiple sclerosis. J Neurol Neurosurg Psychiatry 75:840–846
Diciotti S, Cecchi P, Ginestroni A, Mazzoni LN, Pesaresi I, Lombardo S, Boni E, Cosottini M, Soricelli A, De Stefano N, Mascalchi M (2010) MR-compatible device for monitoring hand tracing and writing tasks in fMRI with an application to healthy subjects. Conc Magn Reson Imaging – Part A 36A:139–152
Jenkinson M, Bannister P, Brady M, Smith S (2002) Improved optimisation for the robust and accurate linear registration and motion correction of brain images. NeuroImage 17:825–841
Smith S (2002) Fast robust automated brain extraction. Hum Brain Mapp 17:143–155
Woolrich W, Ripley BD, Brady JM, Smith SM (2001) Temporal autocorrelation in univariate linear modelling of FMRI data. NeuroImage 14:1370–1386
Worsley KJ (2001) Statistical analysis of activation images. In: Jezzard P, Matthews PM, Smith SM (eds), Functional MRI: an introduction to methods. OUP
Jenkinson M, Smith S (2001) A global optimisation method for robust affine registration of brain images. Med Image Anal 5:143–156
Andersson LR, Jenkinson M, Smith SM (2007) Non-linear optimisation. FMRIB technical report TR07JA1
Andersson LR, Jenkinson M, Smith SM (2007) Non-linear registration, aka spatial normalization. FMRIB technical report TR07JA2
Beckmann C, Jenkinson M, Smith SM (2003) General multi-level linear modelling for group analysis in FMRI. NeuroImage 20:1052–1063
Woolrich MW, Behrens TEJ, Beckmann CF, Jenkinson M, Smith SM (2004) Multi-level linear modelling for FMRI group analysis using Bayesian inference. NeuroImage 21:1732–1747
Woolrich MW (2008) Robust group analysis using outlier inference. NeuroImage 41:286–301
Tzourio-Mazoyer N, Landeau B, Papathanassiou D, Crivello F, Etard O, Delcroix N, Mazoyer B, Joliot M (2002) Automated anatomical labeling of activations in SPM using a macroscopic anatomical parcellation of the MNI MRI single-subject brain. NeuroImage 15:273–289
Ginestroni A, Diciotti S, Cecchi P, Pesaresi I, Tessa C, Giannelli M, Della Nave R, Salvatore E, Salvi F, Dotti MT, Piacentini S, Soricelli A, Cosottini M, De Stefano N, Mascalchi M (2011) Neurodegeneration in Friedreich’s ataxia is associated with a mixed activation pattern of the brain. A fMRI study. Hum Brain Mapp. doi: 10.1002/hbm.21319. Accessed 14 Jun 2011
Schlaug G, Sanes JN, Thangaraj V, Darby DG, Jancke L, Edelman RR, Warach S (1996) Cerebral activations covaries with movement rate. Neuroreport 7:879–883
Rao SM, Bandettini PA, Binder JR, Bobholz JA, Hammeke TA, Stein EA, Hyde JS (1996) Relationship between finger movement rate and functional magnetic resonance signal change in human primary motor cortex. J Cereb Blood Flow Metab 16:1250–1254
Jancke L, Peters M, Schlaug G, Posse S, Steinmetz H, Muller-Gartner HM (1998) Differential magnetic resonance signal change in human sensorimotor cortex to finger movements of different rate of the dominant hand and subdominant hand. Brain Res Cogn Brain Res 6:279–284
Deiber MP, Honda M, Ibanez V, Sadato N, Hallett M (1999) Mesial motor areas in self-initiated versus externally triggered movements examined with fMRI: effect of movement type and rate. J Neurophysiol 81:3065–3077
Riecker A, Wildgruber D, Mathiak K, Grodd W, Ackermann H (2003) Parametric analysis of rate-dependent hemodynamic response functions of cortical and subcortical brain structures during auditorily cued finger tapping: a fMRI study. NeuroImage 18:731–739
Lutz K, Koeneke S, Wustenberg T, Jancke L (2005) Asymmetry of cortical activation during maximum and convenient tapping speed. Neurosci Lett 373:61–66
Cerasa A, Hagberg GE, Peppe A, Bianciardi M, Gioia MC, Costa A, Castriota-Scanderbeg A, Caltagirone C, Sabatini U (2006) Functional changes in the activity of cerebellum and frontostriatal regions during externally and internally timed movement in Parkinson’s disease. Brain Res Bull 71:259–269
Yu H, Sternad D, Corcos DM, Vaillancourt DE (2007) Role of iperactive cerebellum and motor cortex in Parkinson’s disease. NeuroImage 35:222–233
Spraker MB, Prodoehl J, Corcos DM, Comella CL, Vaillancourt DE (2010) Basal ganglia hypoactivity during grip force in drug-naive Parkinson’s disease. Hum Brain Mapp 31:1928–1941
Taniwaki T, Okajama A, Yoshiura T, Togao O, Nakamura Y, Yamasaki T, Ogata K, Shigeto H, Ohyagy Y, Kira J, Tobimatsu S (2006) Functional network of the basal ganglia and cerebellar motor loops in vivo: different activation patterns between self-initiated and externally triggered movements. NeuroImage 31:745–753
Lewis MM, Slagle CG, Smith AB, Truong Y, Bai P, McKeown MJ, Mailman RB, Belger A, Huang X (2007) Task specific influences of Parkinson’s disease on the striato-thalamo-cortical and cerebello-thalamo-cortical motor circuitries. Neuroscience 147:224–235
Conflict of interest
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Supplementary Fig. 1
Areas of activation modulated by frequency (top row) and size variable (bottom row) of “8” figure writing task in the between-group analysis (Z > 5, p = 0.05 corrected). The figure shows multiple areas of cortical activation including SM1, posterior parietal cortex and cerebellum (DOC 412 kb)
Rights and permissions
About this article
Cite this article
Tessa, C., Lucetti, C., Diciotti, S. et al. Hypoactivation of the primary sensorimotor cortex in de novo Parkinson’s disease. Neuroradiology 54, 261–268 (2012). https://doi.org/10.1007/s00234-011-0955-y
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-011-0955-y