Abstract
Introduction
Current endeavors in neuro-oncology include morphological validation of imaging methods by histology, including molecular and immunohistochemical techniques. Diffusion tensor imaging (DTI) is an up-to-date methodology of intracranial diagnostics that has gained importance in studies of neoplasia. Our aim was to assess the feasibility of discriminant analysis applied to histograms of preoperative diffusion tensor imaging-derived images for the prediction of glioma grade validated by histomorphology.
Methods
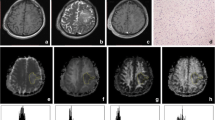
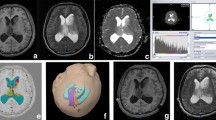
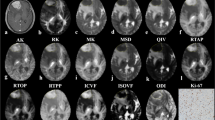
Tumors of 40 consecutive patients included 13 grade II astrocytomas, seven oligoastrocytomas, six grade II oligodendrogliomas, three grade III oligoastrocytomas, and 11 glioblastoma multiformes. Preoperative DTI data comprised: unweighted (B 0) images, fractional anisotropy, longitudinal and radial diffusivity maps, directionally averaged diffusion-weighted imaging, and trace images. Sampling consisted of generating histograms for gross tumor volumes; 25 histogram bins per scalar map were calculated. The histogram bins that allowed the most precise determination of low-grade (LG) or high-grade (HG) classification were selected by multivariate discriminant analysis. Accuracy of the model was defined by the success rate of the leave-one-out cross-validation.
Results
Statistical descriptors of voxel value distribution did not differ between LG and HG tumors and did not allow classification. The histogram model had 88.5% specificity and 85.7% sensitivity in the separation of LG and HG gliomas; specificity was improved when cases with oligodendroglial components were omitted.
Conclusion
Constructing histograms of preoperative radiological images over the tumor volume allows representation of the grade and enables discrimination of LG and HG gliomas which has been confirmed by histopathology.


Similar content being viewed by others
Abbreviations
- ADC:
-
Apparent diffusion coefficient
- CNS:
-
Central nervous system
- DICOM:
-
Digital Imaging and Communications in Medicine
- DTI:
-
Diffusion tensor imaging
- DWI:
-
Diffusion-weighted imaging
- FA:
-
Fractional anisotropy
- GBM:
-
Glioblastoma multiforme
- HG:
-
High grade
- LG:
-
Low grade
- MD:
-
Mean diffusivity (trace/3)
- MDA:
-
Multivariate discriminant analysis
- MRI:
-
Magnetic resonance imaging
- rCBV:
-
Regional cerebral blood volume
- ROI:
-
Region of interest
- SPL:
-
Surgical Planning Laboratory
- TE:
-
Echo time
- TR:
-
Repetition time
- WHO:
-
World Health Organization
References
Louis DH, Ohgaki H, Wiestler OD, Cavanee WK (2007) WHO classification of tumours of the central nervous system. WHO/IARC, Lyon
Ohgaki H, Kleinues P (2005) Population-based studies on incidence, survival rates, and genetic alterations in astrocytic and oligodendroglial gliomas. J Neuropathol Exp Neurol 64:479–489
Cohen N, Weller RO (2007) Who classification of tumours of the central nervous system (4th edition). Neuropathol Appl Neurobiol 33:710–711. doi:10.1111/j.1365-2990.2007.00905.x
Demuth T, Berens ME (2006) Molecular genetics of brain tumors—an overview. In: Newton HB (ed) Handbook of brain tumor chemotherapy, 1st edn. Academic, San Diego, pp 115–122
Le Bihan D, Breton E, Lallemand D, Grenier P, Cabanis E, Laval-Jeantet M (1986) MR imaging of intravoxel incoherent motions: application to diffusion and perfusion in neurologic disorders. Radiology 161:401–407
Basser PJ, Pierpaoli C (1996) Microstructural and physiological features of tissues elucidated by quantitative-diffusion-tensor MRI. J Magn Reson B 111:209–219. doi:10.1006/jmrb.1996.0086
Chun SY, Kun CL, Xuan Y, Xun MJ, Qin W (2005) Diffusion tensor tractography in patients with cerebral tumors: a helpful technique for neurosurgical planning and postoperative assessment. Eur J Radiol 56:197–204
Price SJ (2007) The role of advanced MR imaging in understanding brain tumour pathology. Br J Neurosurg 21:562–575
Rees J (2003) Advances in magnetic resonance imaging of brain tumours. Curr Opin Neurol 16:643–650
Sinha S, Bastin ME, Whittle IR, Wardlaw JM (2002) Diffusion tensor MR imaging of high-grade cerebral gliomas. AJNR Am J Neuroradiol 23:520–527
Tropine A, Vucurevic G, Delani P et al (2004) Contribution of diffusion tensor imaging to delineation of gliomas and glioblastomas. J Magn Reson Imaging 20:905–912. doi:10.1002/jmri.20217
Kono K, Inoue Y, Nakayama K et al (2001) The role of diffusion-weighted imaging in patients with brain tumors. AJNR Am J Neuroradiol 22:1081–1088
Sugahara T, Korogi Y, Kochi M et al (1999) Usefulness of diffusion-weighted MRI with echo-planar technique in the evaluation of cellularity in gliomas. J Magn Reson Imaging 9:53–60
Zimmerman RD (2001) Is there a role for diffusion-weighted imaging in patients with brain tumors or is the “bloom off the rose”? AJNR Am J Neuroradiol 22:1013–1019
Khayal IS, McKnight TR, McGue C et al (2009) Apparent diffusion coefficient and fractional anisotropy of newly diagnosed grade II gliomas. NMR Biomed 22:449–455
Inoue T, Ogasawara K, Beppu T, Ogawa A, Kabasawa H (2005) Diffusion tensor imaging for preoperative evaluation of tumor grade in gliomas. Clin Neurol Neurosurg 107:174–180. doi:10.1016/j.clineuro.2004.06.011
Goebell E, Paustenbach S, Vaeterlein O et al (2006) Low-grade and anaplastic gliomas: differences in architecture evaluated with diffusion-tensor MR imaging. Radiology 239:217–222. doi:10.1148/radiol.2383050059
Murakami R, Hirai T, Sugahara T et al (2009) Grading astrocytic tumors by using apparent diffusion coefficient parameters: superiority of a one- versus two-parameter pilot. Radiology 251:838–845
Verma R, Zacharaki EI, Ou Y et al (2008) Multiparametric tissue characterization of brain neoplasms and their recurrence using pattern classification of MR images. Acad Radiol 15:966–977. doi:10.1016/j.acra.2008.01.029
Emblem KE, Scheie D, Due-Tonnessen P et al (2008) Histogram analysis of MR imaging-derived cerebral blood volume maps: combined glioma grading and identification of low-grade oligodendroglial subtypes. AJNR Am J Neuroradiol 29:1664–1670. doi:10.3174/ajnr.A1182
Tozer DJ, Jäger HR, Danchaivijitr N et al (2007) Apparent diffusion coefficient histograms may predict low-grade glioma subtype. NMR Biomed 20:49–57. doi:10.1002/nbm.1091
Law M, Young R, Babb J, Pollack E, Johnson G (2007) Histogram analysis versus region of interest analysis of dynamic susceptibility contrast perfusion MR imaging data in the grading of cerebral gliomas. AJNR Am J Neuroradiol 28:761–766
Dehmeshki J, Ruto AC, Arridge S, Silver NC, Miller DH, Tofts PS (2001) Analysis of MTR histograms in multiple sclerosis using principal components and multiple discriminant analysis. Magn Reson Med 46:600–609
Dehmeshki J, Van Buchem MA, Bosma GPT, Huizinga TWJ, Tofts PS (2002) Systemic lupus erythematosus: diagnostic application of magnetization transfer ratio histograms in patients with neuropsychiatric symptoms. Radiology 222:722–728
Pieper S, Lorensen B, Schroeder W, Kikinis R (2006) The NA-MIC Kit: ITK, VTK, pipelines, grids and 3D slicer as an open platform for the medical image computing community. Proceedings of the 3rd IEEE International Symposium on Biomedical Imaging: From Nano to Macro 1, pp 698–701
Wang W, Steward CE, Desmond PM (2009) Diffusion tensor imaging in glioblastoma multiforme and brain metastases: the role of p, q, L, and fractional anisotropy. AJNR Am J Neuroradiol 30:203–208
Abramoff MD, Magelhaes PJ, Ram SJ (2004) Image processing with image. J Biophotonics Int 11:36–42
Fisher RA (1936) The use of multiple measurements in taxonomic problems. Ann Eugen 7:179–188
Ripley BD (1997) Pattern recognition and neural networks. Cambridge University Press, Cambridge, p 91
Webb A (1999) Statistical pattern recognition. Oxford University Press, New York, p 6
Fukunaga K, Hayes R (1989) Estimation of classifier performance. IEEE Trans Pattern Anal Mach Intell 11:1087–1097
Stadlbauer A, Ganslandt O, Buslei R et al (2006) Gliomas: histopathologic evaluation of changes in directionality and magnitude of water diffusion at diffusion-tensor MR imaging. Radiology 240:803–810. doi:10.1148/radiol.2403050937
Beppu T, Inoue T, Shibata Y et al (2005) Fractional anisotropy value by diffusion tensor magnetic resonance imaging as a predictor of cell density and proliferation activity of glioblastomas. Surg Neurol 63:56–61. doi:10.1016/j.surneu.2004.02.034
Calvar JA, Meli FJ, Romero C et al (2005) Characterization of brain tumors by MRS, DWI and ki-67 labeling index. J Neurooncol 72:273–280
Higano S, Yun X, Kumabe T et al (2006) Malignant astrocytic tumors: clinical importance of apparent diffusion coefficient in prediction of grade and prognosis. Radiology 241:839–846. doi:10.1148/radiol.2413051276
Mardor Y, Roth Y, Ocherashvilli A et al (2004) Pretreatment prediction of brain tumors’ response to radiation therapy using high b-value diffusion-weighted MRI. Neoplasia 6:136–142
Pope WB, Kim HJ, Huo J et al (2009) Recurrent glioblastoma multiforme: ADC histogram analysis predicts response to bevacizumab treatment. Radiology 252:182–189
Lee HY, Na D et al (2008) Diffusion-tensor imaging for glioma grading at 3-T magnetic resonance imaging: analysis of fractional anisotropy and mean diffusivity. J Comput Assist Tomogr 32:298–303. doi:10.1097/RCT.0b013e318076b44d
Ferda J, Kastner J, Mukenšnabl P et al (2010) Diffusion tensor magnetic resonance imaging of glial brain tumors. Eur J Radiol 74:428–436
Arvinda H, Kesavadas C, Sarma P et al (2009) Glioma grading: sensitivity, specificity, positive and negative predictive values of diffusion and perfusion imaging. J Neurooncol 94:87–96
Acknowledgments
We gratefully acknowledge the financial support of 164/2006 grant of Medical Research Council of Ministry of Health, Hungary. P. Molnár is generously supported by a research grant of VFK Krebsforschung GmbH, Germany.
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Mathematical equations used for generating scalar maps
where λ 1, λ 2, and λ 3 are the three eigenvalues of the diffusion tensor and \( \overline \lambda \) stands for the mean of the three eigenvalues.
where SIDWI is the signal intensity of the voxels on the DWI images, SIT2 means the signal intensity on T2 acquisitions (b 0 images). ADC is the apparent diffusion coefficient while b is a factor reflecting the strength of diffusion weighting.
Rights and permissions
About this article
Cite this article
Jakab, A., Molnár, P., Emri, M. et al. Glioma grade assessment by using histogram analysis of diffusion tensor imaging-derived maps. Neuroradiology 53, 483–491 (2011). https://doi.org/10.1007/s00234-010-0769-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-010-0769-3