Abstract
Introduction
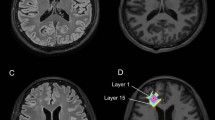
Not uncommonly, differentiating multiple sclerosis (MS) from ischemic cerebral vascular disease is difficult based on conventional magnetic resonance imaging (MRI). We aim to determine whether preferential occult injury in the normal-appearing corpus callosum (NACC) is more severe in patients with MS than symptomatic carotid occlusion by comparing fractional anisotropy (FA) from diffusion tensor imaging (DTI).
Methods
Eighteen patients (eight men, ten women; mean age, 38.6 years) with MS and 32 patients (24 men, eight women; mean age, 64.0 years) with symptomatic unilateral internal carotid occlusion were included. DTI (1.5 T) were performed at corpus callosum which were normal-appearing on fluid-attenuated inversion recovery MRI. Mean FA was obtained from the genu, anterior body, posterior body, and splenium of NACC. Independent-sample t test statistical analysis was performed.
Results
The FA values in various regions of NACC were lower in the MS patients than symptomatic carotid occlusion patients, which was statistically different at the anterior body (0.67 ± 0.12 vs 0.74 ± 0.06, P = 0.009), but not at genu, posterior body, and splenium (0.63 ± 0.09 vs 0.67 ± 0.07, P = 0.13; 0.68 ± 0.09 vs 0.73 ± 0.05, P = 0.07; 0.72 ± 0.09 vs 0.76 ± 0.05, P = 0.13).
Conclusion
MS patients have lower FA in the anterior body of NACC compared to patients with symptomatic carotid occlusion. It suggests that DTI has potential ability to differentiate these two conditions due to the more severe preferential occult injury at the anterior body of NACC in MS.


Similar content being viewed by others
References
Ge Y, Law M, Johnson G, Herbert J, Babb JS, Mannon LJ, Grossman RI (2004) Preferential occult injury of corpus callosum in multiple sclerosis measured by diffusion tensor imaging. J Magn Reson Imaging 20:1–7. doi:10.1002/jmri.20083
Evangelou N, Esiri MM, Smith S, Palace J, Matthews PM (2000) Quantitative pathological evidence for axonal loss in normal appearing white matter in multiple sclerosis. Ann Neurol 47:391–395. doi:10.1002/1531-8249(200003)47:3<391::AID-ANA20>3.0.CO;2-J
Saindane AM, Law M, Ge Y, Johnson G, Babb JS, Grossman RI (2007) Correlation of diffusion tensor and dynamic perfusion MR imaging metrics in normal-appearing corpus callosum: support for primary hypoperfusion in multiple sclerosis. AJNR Am J Neuroradiol 28:767–772
Werring DJ, Toosy AT, Clark CA, Parker GJ, Barker GJ, Miller DH, Thompson AJ (2000) Diffusion tensor imaging can detect and quantify corticospinal tract degeneration after stroke. J Neurol Neurosurg Psychiatry 69:269–2725. doi:10.1136/jnnp.69.2.269
O’Sullivan M, Summers PE, Jones DK, Jarosz JM, Williams SCR, Markus HS (2001) Normal-appearing white matter in ischemic leukoaraiosis: a diffusion tensor MRI study. Neurology 57:2307–2310
O’Sullivan M, Jones DK, Summers PE, Morris RG, Williams SCR, Markus HS (2001) Evidence for cortical “disconnection” as a mechanism of age-related cognitive decline. Neurology 57:632–638
Inglese M, Salvi F, Iannucci G, Manacardi GL, Mascalchi M, Filippi M (2002) Magnetization transfer and diffusion tensor MR imaging of acute disseminated encephalomyelitis. AJNR Am J Neuroradiol 23:267–272
Bisdas S, Bohning DE, Besenski N, Nicholas JS, Rumboldt Z (2008) Reproducibility, interrater agreement, and age-related changes of fractional anisotropy measures at 3 T in healthy subjects: effect of the applied b-value. AJNR Am J Neuroradiol 29:1128–1133. doi:10.3174/ajnr.A1044
Shiraishi A, Hasegawa Y, Okada S, Kimura K, Sawada T, Mizusawa H, Minematsu K (2005) Highly diffusion-sensitized tensor imaging of unilateral cerebral arterial occlusive disease. AJNR Am J Neuroradiol 26:1498–1504
Hines M, Chiu L, McAdams LA, Bentler PM, Lipcamon J (1992) Cognition and the corpus callosum: verbal fluency, visuospatial ability, and language lateralization related to midsagittal surface areas of callosal subregions. Behav Neurosci 106:3–14. doi:10.1037/0735-7044.106.1.3
Abe O, Aoki S, Hayashi N, Yamada H, Kunimatsu A, Mori H, Yoshikawa T, Okubo T, Ohtomo K (2002) Normal aging in the central nervous system: quantitative MR diffusion-tensor analysis. Neurobiol Aging 23:433–441. doi:10.1016/S0197-4580(01)00318-9
Evangelou N, Konz D, Esiri MM, Smith S, Palace J, Matthews PM (2000) Regional axonal loss in the corpus callosum correlates with cerebral white matter lesion volume and distribution in multiple sclerosis. Brain 123(Pt 9):1845–1849. doi:10.1093/brain/123.9.1845
Levin HS, Benavidez DA, Verger-Maestre K, Perachio N, Song J, Mendelsohn DB, Fletcher JM (2000) Reduction of corpus callosum growth after severe traumatic brain injury in children. Neurology 54:647–653
Foong J, Maier M, Clark CA, Barker GJ, Miller DH, Ron MA (2000) Neuropathological abnormalities of the corpus callosum in schizophrenia: a diffusion tensor imaging study. J Neurol Neurosurg Psychiatry 68:242–244. doi:10.1136/jnnp.68.2.242
Werring DJ, Clark CA, Barker GJ, Thompson AJ, Miller DH (1999) Diffusion tensor imaging of lesions and normal-appearing white matter in multiple sclerosis. Neurology 52:1626–1632
Guo A, MacFall J, Provenzale J (2002) Multiple sclerosis: diffusion tensor MR imaging for evaluation of normal-appearing white matter. Radiology 222:729–736. doi:10.1148/radiol.2223010311
Filippi M, Cercignani M, Inglese M, Horsfield M, Comi G (2001) Diffusion tensor magnetic resonance imaging in multiple sclerosis. Neurology 56:304–311
Ozturk A, Sasson AD, Farrell JA, Landman BA, da Motta AC, Aralasmak A, Yousem DM (2008) Regional differences in diffusion tensor imaging measurements: assessment of intrarater and interrater variability. AJNR Am J Neuroradiol 29:1124–1127. doi:10.3174/ajnr.A0998
Filippi M, Campi A, Dousset V, Baratti C, Martinelli V, Canal N, Scotti G, Comi G (1995) A magnetization transfer imaging study of normal-appearing white matter in multiple sclerosis. Neurology 45:478–482
Loevner LA, Grossman RI, Cohen JA, Lexa FJ, Kessler D, Kolson DL (1995) Microscopic disease in normal-appearing white matter on conventional MR images in patients with multiple sclerosis: assessment with magnetization-transfer measurements. Radiology 196:511–515
Tortorella C, Viti B, Bozzali M, Sormani MP, Rizzo G, Gilardi MF, Comi G, Filippi M (2000) A magnetization transfer histogram study of normal-appearing brain tissue in MS. Neurology 54:186–193
Chabriat H, Pappata S, Poupon C, Clark CA, Vahedi K, Poupon F, Mangin JF, Pachot-Clouard M, Jobert A, Le Bihan D, Bousser MG (1999) Clinical severity in CADASIL related to ultrastructural damage in white matter: in vivo study with diffusion tensor MRI. Stroke 30:2637–2643
Werring DJ, Brassat D, Droogan AG, Clark CA, Symms MR, Barker GJ, MacManus DG, Thompson AJ, Miller DH (2000) The pathogenesis of lesions and normal-appearing white matter changes in multiple sclerosis: a serial diffusion MRI study. Brain 123:1667–1676. doi:10.1093/brain/123.8.1667
Bester M, Heesen C, Schippling S, Martin R, Ding XQ, Holst B, Fiehler J (2008) Early anisotropy changes in the corpus callosum of patients with optic neuritis. Neuroradiology 50:549–557. doi:10.1007/s00234-008-0377-7
Bjartmar C, Wujek JR, Trapp BD (2003) Axonal loss in the pathology of MS: consequences for understanding the progressive phase of the disease. J Neurol Sci 206:165–171. doi:10.1016/S0022-510X(02)00069-2
Medana IM, Esiri MM (2003) Axonal damage: a key predictor of outcome in human CNS diseases. Brain 126:515–530. doi:10.1093/brain/awg061
Ryberg C, Rostrup E, Sjöstrand K, Paulson OB, Barkhof F, Scheltens P, van Straaten EC, Fazekas F, Schmidt R, Erkinjuntti T, Wahlund LO, Basile AM, Pantoni L, Inzitari D, Waldemar G, LADIS Study Group (2008) White matter changes contribute to corpus callosum atrophy in the elderly: the LADIS Study. AJNR Am J Neuroradiol 29:1498–1504. doi:10.3174/ajnr.A1169
Chepuri NB, Yen YF, Burdette JH, Li H, Moody DM, Maldjian JA (2002) Diffusion anisotropy in the corpus callosum. AJNR Am J Neuroradiol 23:803–808
Conflict of interest statement
We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Lou, X., Jiang, W., Ma, L. et al. Lower fractional anisotropy at the anterior body of the normal-appearing corpus callosum in multiple sclerosis versus symptomatic carotid occlusion. Neuroradiology 51, 557–561 (2009). https://doi.org/10.1007/s00234-009-0535-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-009-0535-6