Abstract
Introduction
Inherited prion diseases are caused by mutations in the gene which codes for prion protein (PrP), leading to proliferation of abnormal PrP isomers in the brain and neurodegeneration; they include Gerstmann–Sträussler–Scheinker disease (GSS), fatal familial insomnia (FFI) and familial Creutzfeldt–Jakob disease (fCJD).
Methods
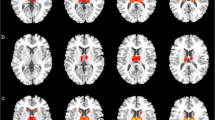
We studied two patients with symptomatic inherited prion disease (P102L) and two pre-symptomatic P102L gene carriers using quantitative magnetic resonance spectroscopy (MRS). Short echo time spectra were acquired from the thalamus, caudate region and frontal white matter, metabolite levels and ratios were measured and z-scores calculated for individual patients relative to age-matched normal controls. MRS data were compared with structural magnetic resonance imaging.
Results
One fCJD case had generalised atrophy and showed increased levels of myo-inositol (MI) in the thalamus (z=3.7). The other had decreased levels of N-acetylaspartate (z=4) and diffuse signal abnormality in the frontal white matter. Both asymptomatic gene carriers had normal imaging, but increased frontal white matter MI (z=4.3, 4.1), and one also had increased MI in the caudate (z=5.3).
Conclusion
Isolated MI abnormalities in asymptomatic gene carriers are a novel finding and may reflect early glial proliferation, prior to significant neuronal damage. MRS provides potential non-invasive surrogate markers of early disease and progression in inherited prion disease.



Similar content being viewed by others
References
Collinge J, Brown J, Hardy J, Mullan M, Rossor MN, Baker H, et al (1992) Inherited prion disease with 144 base pair gene insertion: II: clinical and pathological features. Brain 115:687–710
Collinge J, Palmer MS, Dryden AJ (1991) Genetic predisposition to iatrogenic Creutzfeldt-Jakob disease. Lancet 337:1441–1442
Palmer MS, Dryden AJ, Hughes JT, Collinge J (1991) Homozygous prion protein genotype predisposes to sporadic Creutzfeldt-Jakob disease. Nature 352:340–342
Collinge J (2001) Prion diseases of humans and animals: their causes and molecular basis. Annu Rev Neurosci 24:519–550
Zeidler M, Sellar RJ, Collie DA, et al (2000) The pulvinar sign on magnetic resonance imaging in variant Creutzfeldt-Jakob disease. Lancet 355:1412–1418
Collie DA, Sellar RJ, Zeidler M, Colchester ACF, Knight R, Will RG (2001) MRI of Creutzfeldt-Jakob disease: imaging features and recommended MRI protocol. Clin Radiol 56:726–739
Imaiso Y, Mitsuo K (1998) Gerstmann-Straussler-Scheinker syndrome with a Pro102Leu mutation in the prion protein gene and atypical MRI findings, hyperthermia, tachycardia, and hyperhidrosis. Rinsho Shinkeigaku 38:920–925
Nitrini R, Mendonca RA, Huang N, LeBlanc A, Livramento JA, Marie SK (2001) Diffusion-weighted MRI in two cases of familial Creutzfeldt-Jakob disease. J Neurol Sci 184:163–167
Fox NC, Freeborough PA, Mekkaoui KF, Stevens JM, Rossor MN (1997) Cerebral and cerebellar atrophy on serial magnetic resonance imaging in an initially symptom free subject at risk of familial prion disease. BMJ 4(315):856–857
Bruhn H, Weber T, Thorwirth V, Frahm J (1991) In-vivo monitoring of neuronal loss in Creutzfeldt-Jakob disease by proton magnetic resonance spectroscopy. Lancet 337:1610–1611
Graham GD, Petroff OAC, Blamire AM, Rajkowska G, Goldman-Rakic P, Prichard JW (1993) Proton magnetic resonance spectroscopy in Creutzfeldt-Jakob disease. Neurology 43:2065–2068
Pandya HG, Coley SC, Wilkinson ID, Griffiths PD (2003) Magnetic resonance spectroscopy abnormalities in sporadic and variant Creutzfeldt-Jakob disease. Clin Radiol 58:148–153
Galanaud D, Dormont D, Grabli D, Charles P, Hauw JJ, Lubetzki C, Brandel JP, Marsault C, Cozzone PJ (2002) MR spectroscopic pulvinar sign in a case of variant Creutzfeldt-Jakob disease. J Neuroradiol 29:285–287
Cordery R, MacManus D, Rossor MN, Collinge J, Waldman AD (2003) Short TE proton spectroscopy in variant and familial Creutzfeldt-Jakob disease. Proc ISMRM,11th Scientific Meeting, p 102
Shyu W-C, Lee C-C, Hsu Y-D, et al (1996) Panencephalitic Creutzfeldt-Jakob disease. Unusual presentation of magnetic resonance spectroscopy. J Neurol Sci 138:157–160
Konaka K, Kaido M, Okuda Y, et al (2000) Proton magnetic resonance spectroscopy of a patient with Gerstmann-Straussler-Scheinker disease. Neuroradiology 42:662–665
Provencher SW (1993) Estimation of metabolite concentrations from localized in vivo proton NMR spectra. Magn Reson Med 30:672–679
Behar KL, Boucher R, Fritch W, Manuelidis L (1998) Changes in N-acetylaspartate and myo-inositol detected in the cerebral cortex of hamsters with Creutzfeldt-Jakob disease. Magn Reson Imaging 16:963–968
Cheng LL, Newell K, Mallory AE, Hyman BT (2002) Gonzalez RG quantification of neurons in Alzheimer and control brains with ex vivo high resolution magic angle spinning proton magnetic resonance spectroscopy and stereology. Magn Reson Imaging 20:527–533
Brand A, Richter-Landsberg C, Leibfritz D (1993) Multinuclear NMR studies on the energy metabolism of glial and neuronal cells. Dev Neurosci 15:289–298
Strange K, Emma F, Paredes A, Morrison R (1994) Osmoregulatory changes in myo-inositol content and Na+/myo-inositol cotransport in rat cortical astrocytes. Glia 12:35–43
Valenzuela MJ, Sachdev P (2001) Magnetic resonance spectroscopy in. Neurology 56:592–598
Ironside JW, Bell JE (1997) Florid plaques and new variant Creutzfeldt-Jakob disease. Lancet 350:1475
Huang W, Alexander GE, Daly EM, Shetty HU, Krasuski JS, Rapoport SI, Schapiro MB (1996) High brain myo-inositol levels in the predementia phase of Alzheimer’s disease in adults with Down’s syndrome. Am J Psychiatry 156:1879–1886
Kantarci K, Jack CR, Xu YC, Campeau NG, O’Brien PC, Smith, GE, Ivnik RJ, Boeve, BF, Kokmen E, Tangalos EG, Petersen RC (2000) Regional metabolic patterns in mild cognitive impairment and Alzheimer’s disease. Neurology 55:210–217
Acknowledgements
The authors would like to acknowledge the financial support of the Medical Research Council (grant G9627315), the assistance of colleagues in the Dementia Research Group, MRC Prion Unit and National Prion Clinic, and statistical advice from Hilary Watt, from the Institute of Neurology.
Conflict of interest statement We declare that we have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Waldman, A.D., Cordery, R.J., MacManus, D.G. et al. Regional brain metabolite abnormalities in inherited prion disease and asymptomatic gene carriers demonstrated in vivo by quantitative proton magnetic resonance spectroscopy. Neuroradiology 48, 428–433 (2006). https://doi.org/10.1007/s00234-006-0068-1
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-006-0068-1