Abstract
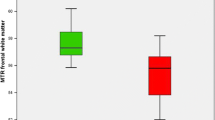
Minimal hepatic encephalopathy (MHE) is frequently diagnosed in patients with liver cirrhosis who do not show overt clinical cirrhosis-associated neurological deficits. This condition manifests primarily with visuo-motor and attention deficits. We studied the association between visuo-motor deficits and magnetic resonance spectroscopic parameters in cingulate grey matter and white matter of centrum semiovale in patients with liver cirrhosis. The data revealed an increase in the glutamate–glutamine/creatine ratio and a decrease in choline/creatine and inositol/creatine ratios in patients with liver cirrhosis. The analysis of the data showed that cirrhosis-associated deterioration of the visuo-motor function significantly correlates with a decrease in the choline/creatine ratio and an increase in N-acetylaspartate/choline in cingulate grey matter but not in the neighbouring white matter. Furthermore, the increase in the glutamate–glutamine/creatine ratio correlated significantly with the increase in the N-acetylaspartate/creatine ratio. These data suggest an association between altered choline, glutamate-glutamine and NAA metabolism in cingulate grey matter and symptoms of MHE, and underline the importance of differentiation between grey and white matter in magnetic resonance spectroscopic studies on patients with cirrhosis-associated brain dysfunction.



Similar content being viewed by others

References
Ferenci P, Lockwood A, Mullen K, Tarter R, Weissenborn K, Blei AT (2002) Hepatic encephalopathy—definition, nomenclature, diagnosis, and quantification: final report of the working party at the 11th World Congresses of Gastroenterology, Vienna, 1998. Hepatology 35:716–721
Lockwood AH (1998) Early detection and treatment of hepatic encephalopathy Curr Opin Neurol 11:663–666
Butterworth RF (2002) Pathophysiology of hepatic encephalopathy: a new look at ammonia. Metab Brain Dis 17:221–227
Weissenborn K, Heidenreich S, Giewekemeyer K, Ruckert N, Hecker H (2003) Memory function in early hepatic encephalopathy. J Hepatol 39: 320–325
Weissenborn K, Heidenreich S, Ennen J, Ruckert N, Hecker H (2001) Attention deficits in minimal hepatic encephalopathy. Metab Brain Dis 16: 13–19
Weissenborn K, Ennen JC, Schomerus H, Ruckert N, Hecker H (2001) Neuropsychological characterization of hepatic encephalopathy. J Hepatol 34:768–773
Groeneweg M, Moerland W, Quero JC, Hop WC, Krabbe PF, Schalm SW (2000) Screening of subclinical hepatic encephalopathy. J Hepatol 32: 748–753
Schomerus H, Hamster W (1998) Neuropsychological aspects of portal-systemic encephalopathy. Metab Brain Dis 13:361–377
Ross BD, Danielsen ER, Bluml S (1996) Proton magnetic resonance spectroscopy: the new gold standard for diagnosis of clinical and subclinical hepatic encephalopathy? Dig Dis 14(Suppl 1):30–39
Naegele T, Grodd W, Viebahn R, Seeger U, Klose U, Seitz D, Kaiser S, Mader I, Mayer J, Lauchart W, Gregor M, Voigt K (2000) MR imaging and (1)H spectroscopy of brain metabolites in hepatic encephalopathy: time-course of renormalization after liver transplantation. Radiology 216:683–691
Kreis R, Ross BD, Farrow NA, Ackerman Z (1992) Metabolic disorders of the brain in chronic hepatic encephalopathy detected with H-1 MR-spectroscopy. Radiology 182:19–27
Ross BD, Jacobson S, Villamil F, Korula J, Kreis R, Ernst T, Shonk T, Moats RA (1994) Subclinical hepatic encephalopathy: proton MR-spectroscopic abnormalities. Radiology 193:457–463
Jalan R, Olde Damink SW, Hayes PC, Wardlaw JM (1999) Diagnosis of hepatic encephalopathy: will in vivo proton MRS play a role? Hepatology 29:1605–1607
Masur H (2000) Skalen und Scores in der Neurologie. Georg Thieme Verlag, Stuttgart, p 497
Lezak DL (1983) Neuropsychological Assessment. Oxford University Press, New York
Spreen O, Strauss E (1991) A Compendium of Neuropsychological Tests. Oxford University Press, New York
Schaaf A, Kessler J, Grond M, Fink GR (1992) Memo-Test. Beltz Test GmbH, Göttingen
Lehrl S (1999) Mehrfach-Wortschatz-Intelligenztest. Spitta Verlag, Berlingen
Knight-Scott J, Haley AP, Rossmiller SR, Farace E, Mai VM, Christopher JM, Manning CA, Simnad VI, Siragy HM (2003) Molality as a unit of measure for expressing 1 H MRS brain metabolite concentrations in vivo. Magn Reson Imaging 21:787–797
Frahm J, Bruhn H, Gyngell ML, Merboldt KD, Hanicke W, Sauter R (1989) Localized high-resolution proton NMR spectroscopy using stimulated echoes: initial applications to human brain in vivo. Magn Reson Med 9:79–93
Michaelis T, Merboldt KD, Hanicke W, Gyngell ML, Bruhn H, Frahm J (1991) On the identification of cerebral metabolites in localized 1 H NMR spectra of human brain in vivo. NMR Biomed 4:90–98
Geissler A, Lock G, Frund R, Held P, Hollerbach S, Andus T, Scholmerich J, Feuerbach S, Holstege A (1997) Cerebral abnormalities in patients with cirrhosis detected by proton magnetic resonance spectroscopy and magnetic resonance imaging. Hepatology 25:48–54
Taylor-Robinson SD, Sargentoni J, Marcus CD, Morgan MY, Bryant DJ (1994) Regional variations in cerebral proton spectroscopy in patients with chronic hepatic encephalopathy. Metab Brain Dis 9:347–359
Cordoba J, Sanpedro F, Alonso J, Rovira A (2002) 1H magnetic resonance in the study of hepatic encephalopathy in humans. Metab Brain Dis 17:415–429
Boesch SM, Schocke M, Burk K, Hollosi P, Fornai F, Aichner FT, Poewe W, Felber S (2001) Proton magnetic resonance spectroscopic imaging reveals differences in spinocerebellar ataxia types 2 and 6. J Magn Reson Imaging 13:553–559
Kantarci K, Reynolds G, Petersen RC, Boeve BF, Knopman DS, Edland SD, Smith GE, Ivnik RJ, Tangalos EG, Jack CR, Jr. (2003) Proton MR-spectroscopy in mild cognitive impairment and Alzheimer disease: comparison of 1.5 and 3 T. AJNR Am J Neuroradiol 24:843–849
Del Sole A, Gambini A, Falini A, Lecchi M, Lucignani G (2002) In vivo neurochemistry with emission tomography and magnetic resonance spectroscopy: clinical applications. Eur Radiol 12:2582–2599
Schocke MFH, Berger T, Felber SR, Wolf C, Deisenhammer F, Kremser C, Seppi K, Aichner FT (2003) Serial contrast-enhanced magnetic resonance imaging and spectroscopic imaging of acute multiple sclerosis lesions under high-dose methylprednisolone therapy. Neuroimage Oct 20:1253–1263
Mechtcheriakov S, Graziadei IW, Mattedi M, Bodner T, Kugener A, Hinterhuber HH, Marksteiner J, Vogel W (2004) Incomplete improvement of visuo-motor deficits in patients with minimal hepatic encephalopathy after liver transplantation. Liver Trans 10:77–83
Pelletier D, Nelson SJ, Grenier D, Lu Y, Genain C, Goodkin DE (2002) 3-D echo planar (1)HMRS imaging in MS: metabolite comparison from supratentorial vs central brain. Magn Reson Imaging 20:599–606
Cordoba J, Hinojosa C, Sampedro F, Alonso J, Rovira A, Quiroga S, Esteban R, Guardia J (2001) Usefulness of magnetic resonance spectroscopy for diagnosis of hepatic encephalopathy in a patient with relapsing confusional syndrome. Dig Dis Sci 46:2451–2455
Catafau AM, Kulisevsky J, Berna L, Pujol J, Martin JC, Otermin P, Balanzo J, Carrio I (2000) Relationship between cerebral perfusion in frontal-limbic-basal ganglia circuits and neuropsychologic impairment in patients with subclinical hepatic encephalopathy. J Nucl Med 41: 405–410
Clarke CE, Lowry M (2001) Systematic review of proton magnetic resonance spectroscopy of the striatum in parkinsonian syndromes. Eur J Neurol 8:573–577
Monfort P, Munoz MD, ElAyadi A, Kosenko E, Felipo V (2002) Effects of hyperammonemia and liver failure on glutamatergic neurotransmission. Metab Brain Dis 17:237–250
Gehl LM, Saab OH, Bzdega T, Wroblewska B, Neale JH (2004) Biosynthesis of NAAG by an enzyme-mediated process in rat central nervous system neurons and glia. J Neurochem 90:989–997
Neale JH, Bzdega T, Wroblewska B (2000) N-Acetylaspartylglutamate: the most abundant peptide neurotransmitter in the mammalian central nervous system. J Neurochem 75:443–452
Pouwels PJ, Frahm J (1997) Differential distribution of NAA and NAAG in human brain as determined by quantitative localized proton MRS. NMR Biomed 10:73–78
Tyson RL, Sutherland GR (1998) Labeling of N-acetylaspartate and N-acetylaspartylglutamate in rat neocortex, hippocampus and cerebellum from [1-13C]glucose. Neurosci Lett 251:181–184
Tkac I, Dirk Keene, Walter C.Low, Rolf Gruetter, Pierre-Gilles Henry (2004) Highly resolved in vivo 1 H NMR spectroscopy of the mouse brain at 9.4 T. Magn Reson Med 52(3):478–484
Rovira A, Grive E, Pedraza S, Rovira A, Alonso J (2001) Magnetization transfer ratio values and proton MR spectroscopy of normal-appearing cerebral white matter in patients with liver cirrhosis. AJNR Am J Neuroradiol 22:1137–1142
Acknowledgements
We are thankful to Dr. G. Kemmler for support in the statistical analysis and to Mrs. Simone Auffinger and Dr. Th. Bodner for their technical assistance. The stipend for M. Mattedi was sponsored by Dr. Kolassa + Merz (Vienna).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Mechtcheriakov, S., Schocke, M., Kugener, A. et al. Chemical shift magnetic resonance spectroscopy of cingulate grey matter in patients with minimal hepatic encephalopathy. Neuroradiology 47, 27–34 (2005). https://doi.org/10.1007/s00234-004-1298-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00234-004-1298-8