Abstract.
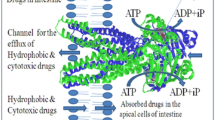
Multidrug resistance to anti-cancer drugs is a major medical problem. Resistance is manifested largely by the product of the human MDR1 gene, P-glycoprotein, an ABC transporter that is an integral membrane protein of 1280 amino acids arranged into two homologous halves, each comprising 6 putative transmembrane α-helices and an ATP binding domain. Despite the plethora of data from site-directed, scanning and domain replacement mutagenesis, epitope mapping and photoaffinity labeling, a clear structural model for P-glycoprotein remains largely elusive. In this report, we propose a new model for P-glycoprotein that is supported by the vast body of previous data. The model comprises 2 membrane-embedded 16-strand β-barrels, attached by short loops to two 6-helix bundles beneath each barrel. Each ATP binding domain contributes 2 β-strands and 1 α-helix to the structure. This model, together with an analysis of the amino acid sequence alignment of P-glycoprotein isoforms, is used to delineate drug binding and translocation sites. We show that the locations of these sites are consistent with mutational, kinetic and labeling data.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 18 February 1998/Revised: 2 September 1998
Rights and permissions
About this article
Cite this article
Jones, P., George, A. A New Structural Model for P-Glycoprotein. J. Membrane Biol. 166, 133–147 (1998). https://doi.org/10.1007/s002329900455
Issue Date:
DOI: https://doi.org/10.1007/s002329900455