Abstract
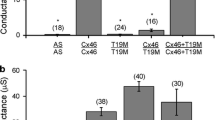
Connexin43 (Cx43) forms gap junctions that couple the granulosa cells of ovarian follicles. In Cx43 knockout mice, follicle growth is restricted as a result of impaired granulosa cell proliferation. We have used these mice to examine the importance of specific Cx43 phosphorylation sites in follicle growth. Serines at residues 255, 262, 279, and 282 are MAP kinase substrates that, when phosphorylated, reduce junctional conductance. Mutant forms of Cx43 were constructed with these serines replaced with amino acids that cannot be phosphorylated. These mutants were transduced into Cx43 knockout ovarian somatic cells that were combined with wild-type oocytes and grafted into immunocompromised female mice permitting follicle growth in vivo. Despite residues 255 or 262 being mutated to prevent their being phosphorylated, recombinant ovaries constructed with these mutants were able to rescue the null phenotype, restoring complete folliculogenesis. In contrast, Cx43 with serine to alanine mutations at both residues 279 and 282 or at all four residues failed to rescue folliculogenesis; the mutant molecules were largely confined to intracellular sites, with few gap junctions. Using an in vitro proliferation assay, we confirmed a decrease in proliferation of granulosa cells expressing the double mutant construct. These results indicate that Cx43 phosphorylation by MAP kinase at serines 279 and 282 occurs in granulosa cells of early follicles and that this is involved in regulating follicle development.






Similar content being viewed by others
References
Ackert CL, Gittens JEI, O’Brien MJ, Eppig JJ, Kidder GM (2001) Intercellular communication via connexin43 gap junctions is required for ovarian folliculogenesis in the mouse. Dev Biol 233:258–270
Beardslee M, Laing J, Beyer E, Saffitz J (1998) Rapid turnover of connexin43 in the adult rat heart. Circ Res 83:629–635
Boassa D, Solan JL, Papas A, Thornton P, Lampe PD, Sosinsky GE (2010) Trafficking and recycling of the connexin43 gap junction protein during mitosis. Traffic 11:1471–1486
Cooper CD, Lampe PD (2002) Casein kinase 1 regulates connexin43 gap junction assembly. J Biol Chem 277:44962–44968
Crow DS, Beyer EC, Paul DL, Kobe SS, Lau AF (1990) Phosphorylation of connexin43 gap junction protein in uninfected and Rous sarcoma virus-transformed mammalian fibroblasts. Mol Cell Biol 10:1754–1763
Eppig JJ, Wigglesworth K (2000) Development of mouse and rat oocytes in chimeric reaggregated ovaries after interspecific exchange of somatic and germ cell components. Biol Reprod 63:1014–1023
Gittens JEI, Kidder GM (2005) Differential contributions of connexin37 and connexin43 to oogenesis revealed in chimeric reaggregated mouse ovaries. J Cell Sci 118:5071–5078
Goodenough DA, Paul DL (2003) Beyond the gap: functions of unpaired connexon channels. Nat Rev Mol Cell Biol 4:285–294
Kanemitsu MY, Jiang W, Eckhart W (1998) Cdc2-mediated phosphorylation of the gap junction protein, connexin43, during mitosis. Cell Growth Differ 9:13–21
Kidder GM, Vanderhyden BC (2010) Bidirectional communication between oocytes and follicle cells: ensuring oocyte developmental competence. Can J Physiol Pharmacol 88:99–413
Laird DW, Puranam KL, Revel JP (1991) Turnover and phosphorylation dynamics of connexin43 gap junction protein in cultured cardiac myocytes. Biochem J 273:67–72
Lampe PD (1994) Analyzing phorbol ester effects on gap junction communication: a dramatic inhibition of assembly. J Cell Biol 127:1895–1905
Lampe PD, Lau AF (2000) Regulation of gap junctions by phosphorylation. Arch Biochem Biophys 384:205–215
Lampe PD, Lau AF (2004) The effects of connexin phosphorylation on gap junctional communication. Int J Biochem Cell Biol 36:1171–1186
Lampe PD, Kurata WE, Warn-Cramer B, Lau AF (1998) Formation of a distinct connexin43 phosphoisoform in mitotic cells is dependent upon p34cdc2 kinase. J Cell Sci 111:833–841
Lampe PD, TenBroek EM, Burt JM, Kurata WE, Johnson RG, Lau AF (2000) Phosphorylation of connexin43 on serine368 by protein kinase C regulates gap junctional communication. J Cell Biol 126:1503–1512
Leithe E, Rivedal E (2004) Epidermal growth factor regulates ubiquitination, internalization and proteasome-dependent degradation of connexin43. J Cell Sci 117:1211–1220
Leykauf K, Dürst M, Alonso A (2003) Phosphorylation and subcellular distribution of connexin43 in normal and stressed cells. Cell Tiss Res 311:23–30
Maass K, Ghanem A, Kim J-S, Saathoff M, Urschel S, Kirfel G, Grümmer R, Kretz M, Lewalter T, Tiemann K, Winterhager E, Herzog V, Willecke K (2004) Defective epidermal barrier in neonatal mice lacking the C-terminal region of connexin43. Mol Biol Cell 15:4597–4608
Maass K, Shibayama J, Chase SE, Willecke K, Delmar M (2007) C-terminal truncation of connexin43 changes number, size, and localization of cardiac gap junction plaques. Circ Res 101:1283–1291
Musil LS, Beyer EC, Goodenough DA (1990) Expression of the gap junction protein connexin43 in embryonic chick lens: molecular cloning, ultrastructural localization, and post-translational phosphorylation. J Membr Biol 116:163–175
Norris RP, Freudzon M, Mehlmann LM, Cowan AE, Simon AM, Paul DL, Lampe PD, Jaffe LA (2008) Luteinizing hormone causes MAP kinase-dependent phosphorylation and closure of connexin43 gap junctions in mouse ovarian follicles: one of two paths to meiotic resumption. Development 135:3229–3238
Park DJ, Wallick CJ, Martyn KD, Lau AF, Jin C, Warn-Cramer BJ (2007) Akt phosphorylates Connexin43 on Ser373, a “mode-1” binding site for 14-3-3. Cell Commun Adhes 14:211–226
Reaume AG, De Sousa PA, Kulkarni S, Langille BL, Zhu D, Davies TC, Juneja SC, Kidder GM, Rossant J (1995) Cardiac malformation in neonatal mice lacking connexin43. Science 267:831–1834
Saez JC, Nairn AC, Czernik AJ, Fishman GI, Spray DC, Hertzberg EL (1997) Phosphorylation of connexin43 and the regulation of neonatal rat cardiac myocyte gap junctions. J Mol Cell Cardiol 29:2131–2145
Sela-Abramovich S, Chorev E, Galiani D, Dekel N (2005) Mitogen-activated protein kinase mediates luteinizing hormone-induced breakdown of communication and oocyte maturation in rat ovarian follicles. Endocrinology 146:1236–1244
Sela-Abramovich S, Galiani D, Nevo N, Dekel N (2008) Inhibition of rat oocyte maturation and ovulation by nitric oxide: mechanism of action. Biol Reprod 78:1111–1118
Solan JL, Lampe PD (2008) Connexin43 in LA-25 cells with active v-src is phosphorylated on Y247, Y265, S262, S279/282 and S368 via multiple signaling pathways. Cell Comm Adhes 15:75–84
Solan JL, Lampe PD (2009) Connexin43 phosphorylation: structural changes and biological effects. Biochem J 419:261–272
Solan JL, Marquez-Rosado L, Sorgen PL, Thornton PJ, Gafken PR, Lampe PD (2007) Phosphorylation of Cx43 at S365 is a gatekeeper event that changes the structure of Cx43 and prevents downregulation by PKC. J Cell Biol 179:1301–1309
Sosinsky GE, Solan JL, Gaietta GM, Ngan L, Mackey M, Lampe PD (2007) The C-terminus of connexin43 adopts different conformations in the Golgi and gap junction as detected with structure specific antibodies. Biochem J 408:375–385
Swenson KI, Piwnica-Worms H, McNamee H, Paul DL (1990) Tyrosine phosphorylation of the gap junction protein connexin43 is required for pp 60src-induced inhibition of communication. Cell Regul 1:989–1002
Tong D, Gittens JEI, Kidder GM, Bai D (2006) Patch clamp study reveals that the importance of connexin43-mediated gap junctional communication for ovarian folliculogenesis is strain-specific in the mouse. Am J Physiol Cell Physiol 290:290–C297
Tong D, Li TY, Naus KE, Bai D, Kidder GM (2007) In vivo analysis of undocked connexin43 gap junction hemichannels in ovarian granulosa cells. J Cell Sci 120:4016–4024
Tong D, Colley D, Thoo R, Li TY, Plante I, Laird DW, Bai D, Kidder GM (2009) Oogenesis defects in a mutant mouse model of oculodentodigital dysplasia. Dis Models Mech 2:157–167
Warn-Cramer BJ, Lampe PD, Kurata WE, Kanemitsu MY, Loo LWM, Eckhart W, Lau AF (1996) Characterization of the MAP kinase phosphorylation sites on the connexin43 gap junction protein. J Biol Chem 271:3779–3786
Warn-Cramer BJ, Cottrell GT, Burt JM, Lau AF (1998) Regulation of connexin43 gap junctional intercellular communication by mitogen-activated protein kinase. J Biol Chem 273:9188–9196
Acknowledgments
Supported in part by an operating grant to G.M.K. and a Postdoctoral Fellowship to P.W.D. from the Canadian Institutes of Health Research and grant GM55632 to P.D.L. from the U.S. National Institutes of Health. We thank Kevin Barr, Dan Li, and Tony Y. Li for their expert technical assistance and the University of Western Ontario Health Sciences Animal Facility staff for providing mouse care.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Dyce, P.W., Norris, R.P., Lampe, P.D. et al. Phosphorylation of Serine Residues in the C-terminal Cytoplasmic Tail of Connexin43 Regulates Proliferation of Ovarian Granulosa Cells. J Membrane Biol 245, 291–301 (2012). https://doi.org/10.1007/s00232-012-9450-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-012-9450-6