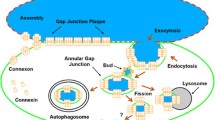
Abstract
Gap junction channels composed of connexins connect cells, allowing intercellular communication. Their cellular assembly involves a unique quality-control pathway. Some connexins [including connexin43 (Cx43) and Cx46] oligomerize in the trans-Golgi network following export of stabilized monomers from the endoplasmic reticulum (ER). In contrast, other connexins (e.g., Cx32) oligomerize early in the secretory pathway. Amino acids near the cytoplasmic aspect of the third transmembrane domain have previously been shown to determine this difference in assembly sites. Here, we characterized the oligomerization of two connexins expressed prominently in the vasculature, Cx37 and Cx40, using constructs containing a C-terminal dilysine-based ER retention/retrieval signal (HKKSL) or treatment with brefeldin A to block ER vesicle trafficking. Both methods led to intracellular retention of connexins, since the cells lacked gap junction plaques. Retention of Cx40 in the ER prevented it from oligomerizing, comparable to Cx43. By contrast, ER-retained Cx37 was partially oligomerized. Replacement of two amino acids near the third transmembrane domain of Cx43 (L152 and R153) with the corresponding amino acids from Cx37 (M152 and G153) resulted in early oligomerization in the ER. Thus, residues that allow Cx37 to oligomerize early in the secretory pathway could restrict its interactions with coexpressed Cx40 or Cx43 by favoring homomeric oligomerization, providing a structural basis for cells to produce gap junction channels with different connexin composition.







Similar content being viewed by others
References
Beyer EC, Berthoud VM (2009) The family of connexin genes. In: Harris AL, Locke D (eds) Connexins: a guide. Humana Press, New York, pp 3–26
Beyer EC, Gemel J, Martinez A, Berthoud VM, Valiunas V, Moreno AP, Brink PR (2001) Heteromeric mixing of connexins: compatibility of partners and functional consequences. Cell Commun Adhes 8:199–204
Brisset AC, Isakson BE, Kwak BR (2009) Connexins in vascular physiology and pathology. Antioxid Redox Signal 11:267–282
Chen J, Anderson JB, DeWeese-Scott C, Fedorova ND, Geer LY, He S, Hurwitz DI, Jackson JD, Jacobs AR, Lanczycki CJ, Liebert CA, Liu C, Madej T, Marchler-Bauer A, Marchler GH, Mazumder R, Nikolskaya AN, Rao BS, Panchenko AR, Shoemaker BA, Simonyan V, Song JS, Thiessen PA, Vasudevan S, Wang Y, Yamashita RA, Yin JJ, Bryant SH (2003) MMDB: Entrez’s 3D-structure database. Nucleic Acids Res 31:474–477
Cottrell GT, Burt JM (2005) Functional consequences of heterogeneous gap junction channel formation and its influence in health and disease. Biochim Biophys Acta 1711:126–141
Das Sarma J, Wang F, Koval M (2002) Targeted gap junction protein constructs reveal connexin-specific differences in oligomerization. J Biol Chem 277:20911–20918
Das S, Smith TD, Das Sarma J, Ritzenthaler JD, Maza J, Kaplan BE, Cunningham LA, Suaud L, Hubbard MJ, Rubenstein RC, Koval M (2009) ERp29 restricts connexin43 oligomerization in the endoplasmic reticulum. Mol Biol Cell 20:2593–2604
Das Sarma J, Das S, Koval M (2005) Regulation of connexin43 oligomerization is saturable. Cell Commun Adhes 12:237–247
Das Sarma J, Kaplan BE, Willemsen D, Koval M (2008) Identification of rab20 as a potential regulator of connexin43 trafficking. Cell Commun Adhes 15:65–74
Daugherty BL, Ward C, Smith T, Ritzenthaler JD, Koval M (2007) Regulation of heterotypic claudin compatibility. J Biol Chem 282:30005–30013
Figueroa XF, Isakson BE, Duling BR (2004) Connexins: gaps in our knowledge of vascular function. Physiology (Bethesda) 19:277–284
Gabriels JE, Paul DL (1998) Connexin43 is highly localized to sites of disturbed flow in rat aortic endothelium but connexin37 and connexin40 are more uniformly distributed. Circ Res 83:636–643
Gemel J, Valiunas V, Brink PR, Beyer EC (2004) Connexin43 and connexin26 form gap junctions, but not heteromeric channels in co-expressing cells. J Cell Sci 117:2469–2480
Gong X, Agopian K, Kumar NM, Gilula NB (1999) Genetic factors influence cataract formation in α3 connexin knockout mice. Dev Genet 24:27–32
Heberlein KR, Straub AC, Isakson BE (2009) The myoendothelial junction: breaking through the matrix? Microcirculation 16:307–322
Hirst-Jensen BJ, Sahoo P, Kieken F, Delmar M, Sorgen PL (2007) Characterization of the pH-dependent interaction between the gap junction protein connexin43 carboxyl terminus and cytoplasmic loop domains. J Biol Chem 282:5801–5813
Hoehenwarter W, Tang Y, Ackermann R, Pleissner KP, Schmid M, Stein R, Zimny-Arndt U, Kumar NM, Jungblut PR (2008) Identification of proteins that modify cataract of mouse eye lens. Proteomics 8:5011–5024
Isakson BE, Duling BR (2005) Heterocellular contact at the myoendothelial junction influences gap junction organization. Circ Res 97:44–51
Isakson BE, Best AK, Duling BR (2008) Incidence of protein on actin bridges between endothelium and smooth muscle in arterioles demonstrates heterogeneous connexin expression and phosphorylation. Am J Physiol Heart Circ Physiol 294:H2898–H2904
Johnson R, Hammer M, Sheridan J, Revel JP (1974) Gap junction formation between reaggregated Novikoff hepatoma cells. Proc Natl Acad Sci USA 71:4536–4540
Johnson RG, Reynhout JK, Tenbroek EM, Quade BJ, Yasumura T, Davidson KG, Sheridan JD, Rash JE (2012) Gap junction assembly: roles for the formation plaque and regulation by the C-terminus of connexin43. Mol Biol Cell 23:71–86
Johnstone S, Isakson B, Locke D (2009) Biological and biophysical properties of vascular connexin channels. Int Rev Cell Mol Biol 278:69–118
Kanter HL, Saffitz JE, Beyer EC (1994) Molecular cloning of two human cardiac gap junction proteins, connexin40 and connexin45. J Mol Cell Cardiol 26:861–868
Koval M (2006) Pathways and control of connexin oligomerization. Trends Cell Biol 16:159–166
Koval M, Geist ST, Westphale EM, Kemendy AE, Civitelli R, Beyer EC, Steinberg TH (1995) Transfected connexin45 alters gap junction permeability in cells expressing endogenous connexin43. J Cell Biol 130:987–995
Koval M, Harley JE, Hick E, Steinberg TH (1997) Connexin46 is retained as monomers in a trans-Golgi compartment of osteoblastic cells. J Cell Biol 137:847–857
Lagree V, Brunschwig K, Lopez P, Gilula NB, Richard G, Falk MM (2003) Specific amino-acid residues in the N-terminus and TM3 implicated in channel function and oligomerization compatibility of connexin43. J Cell Sci 116:3189–3201
Laird DW (2006) Life cycle of connexins in health and disease. Biochem J 394:527–543
Laird DW (2010) The gap junction proteome and its relationship to disease. Trends Cell Biol 20:92–101
Maeda S, Nakagawa S, Suga M, Yamashita E, Oshima A, Fujiyoshi Y, Tsukihara T (2009) Structure of the connexin 26 gap junction channel at 3.5 Å resolution. Nature 458:597–602
Martínez AD, Maripillán J, Acuña R, Minogue PJ, Berthoud VM, Beyer EC (2011) Different domains are critical for oligomerization compatibility of different connexins. Biochem J 436:35–43
Maza J, Mateescu M, Sarma JD, Koval M (2003) Differential oligomerization of endoplasmic reticulum-retained connexin43/connexin32 chimeras. Cell Commun Adhes 10:319–322
Maza J, Das Sarma J, Koval M (2005) Defining a minimal motif required to prevent connexin oligomerization in the endoplasmic reticulum. J Biol Chem 280:21115–21121
Minogue PJ, Liu X, Ebihara L, Beyer EC, Berthoud VM (2005) An aberrant sequence in a connexin46 mutant underlies congenital cataracts. J Biol Chem 280:40788–40795
Musil LS, Goodenough DA (1993) Multisubunit assembly of an integral plasma membrane channel protein, gap junction connexin43, occurs after exit from the ER. Cell 74:1065–1077
Paznekas WA, Karczeski B, Vermeer S, Lowry RB, Delatycki M, Laurence F, Koivisto PA, Van Maldergem L, Boyadjiev SA, Bodurtha JN, Jabs EW (2009) GJA1 mutations, variants, and connexin 43 dysfunction as it relates to the oculodentodigital dysplasia phenotype. Hum Mutat 30:724–733
Reed KE, Westphale EM, Larson DM, Wang HZ, Veenstra RD, Beyer EC (1993) Molecular cloning and functional expression of human connexin37, an endothelial cell gap junction protein. J Clin Invest 91:997–1004
Severs NJ, Rothery S, Dupont E, Coppen SR, Yeh HI, Ko YS, Matsushita T, Kaba R, Halliday D (2001) Immunocytochemical analysis of connexin expression in the healthy and diseased cardiovascular system. Microsc Res Tech 52:301–322
Wang Y, Addess KJ, Chen J, Geer LY, He J, He S, Lu S, Madej T, Marchler-Bauer A, Thiessen PA, Zhang N, Bryant SH (2007) MMDB: annotating protein sequences with Entrez’s 3D-structure database. Nucleic Acids Res 35:D298–D300
Wittig I, Braun HP, Schagger H (2006) Blue native PAGE. Nat Protoc 1:418–428
Yeh HI, Rothery S, Dupont E, Coppen SR, Severs NJ (1998) Individual gap junction plaques contain multiple connexins in arterial endothelium. Circ Res 83:1248–1263
Acknowledgments
This work was supported by the Emory Alcohol and Lung Biology Center/National Institutes of Health grants P50-AA013757 (M. K.), R01-HL083120 (M. K.), RO1-HL59199 (E. C. B.) and RO1-EY08368 (E. C. B.) and by the Emory University Research Committee (M. K.).
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Smith, T.D., Mohankumar, A., Minogue, P.J. et al. Cytoplasmic Amino Acids within the Membrane Interface Region Influence Connexin Oligomerization. J Membrane Biol 245, 221–230 (2012). https://doi.org/10.1007/s00232-012-9443-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-012-9443-5