Abstract
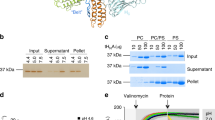
Botulinum neurotoxins (BoNTs) undergo low pH-triggered membrane insertion, resulting in the translocation of their light (catalytic) chains into the cytoplasm. The T (translocation) domain of the BoNT heavy chain is believed to carry out translocation. Here, the behavior of isolated T domain from BoNT type A has been characterized, both in solution and when associated with model membranes. When BoNT T domain prepared in the detergent dodecylmaltoside was diluted into aqueous solution, it exhibited a low pH-dependent conformational change below pH 6. At low pH the T domain associated with, and formed pores within, model membrane vesicles composed of 30 mol% dioleoylphosphatidylglycerol/70 mol% dioleoylphosphatidylcholine. Although T domain interacted with vesicles at low (50 mM) and high (400 mM) NaCl concentrations, the interaction required much less lipid at low salt. However, even at high lipid concentrations pore formation was much more pronounced at low NaCl concentrations than at high NaCl concentration. Increasing salt concentration after insertion in the presence of 50 mM NaCl did not decrease pore formation. A similar effect of NaCl concentration upon pore formation was observed in vesicles composed solely of dioleoylphosphatidylcholine, showing that the effect of NaCl did not solely involve modulation of electrostatic interactions between protein and anionic lipids. These results indicate that some feature of membrane-bound T domain tertiary structure critical for pore formation is highly dependent upon salt concentration.







Similar content being viewed by others
References
Baldwin MR, Barbieri JT (2007) Association of botulinum neurotoxin serotypes a and b with synaptic vesicle protein complexes. Biochemistry 46:3200–3210
Blaustein RO, Germann WJ, Finkelstein A, DasGupta BR (1987) The N-terminal half of the heavy chain of botulinum type A neurotoxin forms channels in planar phospholipid bilayers. FEBS Lett 226:115–120
Blewitt MG, Chung LA, London E (1985) Effect of pH on the conformation of diphtheria toxin and its implications for membrane penetration. Biochemistry 24:5458–5464
Brunger AT, Breidenbach MA, Jin R, Fischer A, Santos JS, Montal M (2007) Botulinum neurotoxin heavy chain belt as an intramolecular chaperone for the light chain. PLoS Pathog 3:1191–1194
Chenal A, Savarin P, Nizard P, Guillain F, Gillet D, Forge V (2002) Membrane protein insertion regulated by bringing electrostatic and hydrophobic interactions into play. A case study with the translocation domain of diphtheria toxin. J Biol Chem 277:43425–43432
Choe S, Bennett MJ, Fujii G, Curmi PM, Kantardjieff KA, Collier RJ, Eisenberg D (1992) The crystal structure of diphtheria toxin. Nature 357:216–222
Emans N, Biwersi J, Verkman AS (1995) Imaging of endosome fusion in BHK fibroblasts based on a novel fluorimetric avidin-biotin binding assay. Biophys J 69:716–728
Fischer A, Montal M (2007) Single molecule detection of intermediates during botulinum neurotoxin translocation across membranes. Proc Natl Acad Sci USA 104:10447–10452
Fu FN, Busath DD, Singh BR (2002) Spectroscopic analysis of low pH and lipid-induced structural changes in type A botulinum neurotoxin relevant to membrane channel formation and translocation. Biophys Chem 99:17–29
Galloux M, Vitrac H, Montagner C, Raffestin S, Popoff MR, Chenal A, Forge V, Gillet D (2008) Membrane interaction of botulinum neurotoxin A T domain: the belt region is a regulatory loop for membrane interaction. J Biol Chem 283:27668–27676
Hammond K, Caputo GA, London E (2002) Interaction of the membrane-inserted diphtheria toxin T domain with peptides and its possible implications for chaperone-like T domain behavior. Biochemistry 41:3243–3253
Hanson MA, Stevens RC (2000) Cocrystal structure of synaptobrevin-II bound to botulinum neurotoxin type B at 2.0 Å resolution. Nat Struct Biol 7:687–692
Kachel K, Ren J, Collier RJ, London E (1998) Identifying transmembrane states and defining the membrane insertion boundaries of hydrophobic helices in membrane-inserted diphtheria toxin T domain. J Biol Chem 273:22950–22956
Koehler TM, Collier RJ (1991) Anthrax toxin protective antigen: low-pH-induced hydrophobicity and channel formation in liposomes. Mol Microbiol 5:1501–1506
Koriazova LK, Montal M (2003) Translocation of botulinum neurotoxin light chain protease through the heavy chain channel. Nat Struct Biol 10:13–18
Krantz BA, Finkelstein A, Collier RJ (2006) Protein translocation through the anthrax toxin transmembrane pore is driven by a proton gradient. J Mol Biol 355:968–979
Kumaran D, Eswaramoorthy S, Furey W, Navaza J, Sax M, Swaminathan S (2009) Domain organization in Clostridium botulinum neurotoxin type E is unique: its implication in faster translocation. J Mol Biol 386:233–245
Lacy DB, Tepp W, Cohen AC, DasGupta BR, Stevens RC (1998) Crystal structure of botulinum neurotoxin type A and implications for toxicity. Nat Struct Biol 5:898–902
Lai B, Zhao G, London E (2008) Behavior of the deeply inserted helices in diphtheria toxin T domain: helices 5, 8, and 9 interact strongly and promote pore formation, while helices 6/7 limit pore formation. Biochemistry 47:4565–4574
Lalli G, Bohnert S, Deinhardt K, Verastegui C, Schiavo G (2003) The journey of tetanus and botulinum neurotoxins in neurons. Trends Microbiol 11:431–437
London E (1992) Diphtheria toxin: membrane interaction and membrane translocation. Biochim Biophys Acta 1113:25–51
Malenbaum SE, Collier RJ, London E (1998) Membrane topography of the T domain of diphtheria toxin probed with single tryptophan mutants. Biochemistry 37:17915–17922
Montecucco C, Schiavo G, Dasgupta BR (1989) Effect of pH on the interaction of botulinum neurotoxins A, B and E with liposomes. Biochem J 259:47–53
Nassi S, Collier RJ, Finkelstein A (2002) PA63 channel of anthrax toxin: an extended beta-barrel. Biochemistry 41:1445–1450
Nicol F, Nir S, Szoka FC Jr (1999) Orientation of the pore-forming peptide GALA in POPC vesicles determined by a BODIPY-avidin/biotin binding assay. Biophys J 76:2121–2141
Puhar A, Johnson EA, Rossetto O, Montecucco C (2004) Comparison of the pH-induced conformational change of different clostridial neurotoxins. Biochem Biophys Res Commun 319:66–71
Ren J, Kachel K, Kim H, Malenbaum SE, Collier RJ, London E (1999) Interaction of diphtheria toxin T domain with molten globule-like proteins and its implications for translocation. Science 284:955–957
Rosconi MP, London E (2002) Topography of helices 5–7 in membrane-inserted diphtheria toxin T domain: identification and insertion boundaries of two hydrophobic sequences that do not form a stable transmembrane hairpin. J Biol Chem 277:16517–16527
Rosconi MP, Zhao G, London E (2004) Analyzing topography of membrane-inserted diphtheria toxin T domain using BODIPY-streptavidin: at low pH, helices 8 and 9 form a transmembrane hairpin but helices 5–7 form stable nonclassical inserted segments on the cis side of the bilayer. Biochemistry 43:9127–9139
Schiavo G, Boquet P, Dasgupta BR, Montecucco C (1990) Membrane interactions of tetanus and botulinum neurotoxins: a photolabelling study with photoactivatable phospholipids. J Physiol (Paris) 84:180–187
Schiavo G, Matteoli M, Montecucco C (2000) Neurotoxins affecting neuroexocytosis. Physiol Rev 80:717–766
Sharpe JC, London E (1999) Diphtheria toxin forms pores of different sizes depending on its concentration in membranes: probable relationship to oligomerization. J Membr Biol 171:209–221
Studier FW (2005) Protein production by auto-induction in high density shaking cultures. Protein Expr Purif 41:207–234
Swaminathan S, Eswaramoorthy S (2000) Structural analysis of the catalytic and binding sites of Clostridium botulinum neurotoxin B. Nat Struct Biol 7:693–699
Wang Y, Kachel K, Pablo L, London E (1997a) Use of Trp mutations to evaluate the conformational behavior and membrane insertion of A and B chains in whole diphtheria toxin. Biochemistry 36:16300–16308
Wang Y, Malenbaum SE, Kachel K, Zhan H, Collier RJ, London E (1997b) Identification of shallow and deep membrane-penetrating forms of diphtheria toxin T domain that are regulated by protein concentration and bilayer width. J Biol Chem 272:25091–25098
Yowler BC, Schengrund CL (2004) Botulinum neurotoxin A changes conformation upon binding to ganglioside GT1b. Biochemistry 43:9725–9731
Yowler BC, Kensinger RD, Schengrund CL (2002) Botulinum neurotoxin A activity is dependent upon the presence of specific gangliosides in neuroblastoma cells expressing synaptotagmin I. J Biol Chem 277:32815–32819
Zakharov SD, Lindeberg M, Griko Y, Salamon Z, Tollin G, Prendergast FG, Cramer WA (1998) Membrane-bound state of the colicin E1 channel domain as an extended two-dimensional helical array. Proc Natl Acad Sci USA 95:4282–4287
Zakharov SD, Lindeberg M, Cramer WA (1999) Kinetic description of structural changes linked to membrane import of the colicin E1 channel protein. Biochemistry 38:11325–11332
Zhan H, Choe S, Huynh PD, Finkelstein A, Eisenberg D, Collier RJ (1994) Dynamic transitions of the transmembrane domain of diphtheria toxin: disulfide trapping and fluorescence proximity studies. Biochemistry 33:11254–11263
Zhao G, London E (2005) Behavior of diphtheria toxin T domain containing substitutions that block normal membrane insertion at Pro345 and Leu307: control of deep membrane insertion and coupling between deep insertion of hydrophobic subdomains. Biochemistry 44:4488–4498
Acknowledgment
This research was supported by awards from DTRA BO742081 under DOE prime contract DEAC02-98CH10886 with Brookhaven National Laboratory to S. S. and an SBU-BNL Seed Grant to E. L.
Author information
Authors and Affiliations
Corresponding authors
Additional information
Bing Lai and Rakhi Agarwal both contributed equally to this work.
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Lai, B., Agarwal, R., Nelson, L.D. et al. Low pH-Induced Pore Formation by the T Domain of Botulinum Toxin Type A is Dependent upon NaCl Concentration. J Membrane Biol 236, 191–201 (2010). https://doi.org/10.1007/s00232-010-9292-z
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-010-9292-z