Abstract
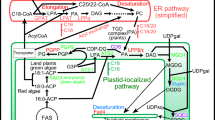
Desaturases that introduce double bonds into the fatty acids are involved in the adaptation of membrane fluidity to changes in the environment. Besides, polyunsaturated fatty acids (PUFAs) are increasingly recognized as important pharmaceutical and nutraceutical compounds. To successfully engineer organisms with increased stress tolerance or the ability to synthesize valuable PUFAs, detailed knowledge about the complexity of the desaturase family as well as understanding of the coevolution of desaturases and their cytochrome b5 electron donors is needed. We have constructed phylogenies of several hundred desaturase sequences from animals, plants, fungi and bacteria and of the cytochrome b5 domains that are fused to some of these enzymes. The analysis demonstrates the existence of three major desaturase acyl-CoA groups that share few similarities. Our results indicate that the fusion of Δ6-desaturase-like enzymes with their cytochrome b5 electron donor was a single event that took place in the common ancestor of all eukaryotes. We also propose the Δ6-desaturase-like enzymes as the most probable donor of the cytochrome b5 domain found in fungal Δ9-desaturases and argue that the recombination most likely happened soon after the separation of the animal and fungal ancestors. These findings answer some of the previously unresolved questions and contribute to the quickly expanding field of research on desaturases.





Similar content being viewed by others
References
Abe T, Sakuradani E, Asano T, Kanamaru H, Shimizu S (2006) Functional characterization of Delta9 and omega9 desaturase genes in Mortierella alpina 1S-4 and its derivative mutants. Appl Microbiol Biotechnol 70:711–719
Aguilar PS, de Mendoza D (2006) Control of fatty acid desaturation: a mechanism conserved from bacteria to humans. Mol Microbiol 62:1507–1514
Altschul SF, Madden TL, Shaffer AA, Zhang Z, Miller W, Lipman DJ (1997) Gapped BLAST and PSI-BLAST: a new generation of protein database search programs. Nucleic Acids Res 25:3389–3402
Anisimova M, Gascuel O (2006) Approximate likelihood-ratio test for branches: a fast, accurate, and powerful alternative. Syst Biol 55:539–552
Crooks GE, Hon G, Chandonia JM, Brenner SE (2004) WebLogo: a sequence logo generator. Genome Res 14:1188–1190
Dailey HA, Strittmatter P (1980) Characterization of the interaction of amphipathic cytochrome b5 with stearyl coenzyme A desaturase and NADPH:cytochrome P-450 reductase. J Biol Chem 255:5184–5189
Fang S, Ting CT, Lee CR, Chu KH, Wang CC, Tsaur SC (2009) Molecular evolution and functional diversification of fatty acid desaturases after recurrent gene duplication in Drosophila. Mol Biol Evol 26:1447–1456
Gostinčar C, Turk M, Gunde-Cimerman N (2009a) Environmental impacts on fatty acid composition of fungal membranes. In: Misra JK, Deshmukh SK (eds) Fungi from different environments. Science Publishers, Enfield, NH, pp 278–327
Gostinčar C, Turk M, Plemenitaš A, Gunde-Cimerman N (2009b) The expressions of D9-, D12-desaturases and an elongase by the extremely halotolerant black yeast Hortaea werneckii are salt dependent. FEMS Yeast Res 9:247–256
Guillou H, D’Andrea S, Rioux V, Barnouin R, Dalaine S, Pedrono F, Jan S, Legrand P (2004) Distinct roles of endoplasmic reticulum cytochrome b5 and fused cytochrome b5-like domain for rat Delta6-desaturase activity. J Lipid Res 45:32–40
Guindon S, Gascuel O (2003) A simple, fast, and accurate algorithm to estimate large phylogenies by maximum likelihood. Syst Biol 52:696–704
Hashimoto K, Yoshizawa AC, Okuda S, Kuma K, Goto S, Kanehisa M (2008) The repertoire of desaturases and elongases reveals fatty acid variations in 56 eukaryotic genomes. J Lipid Res 49:183–191
Hazel JR, Williams EE (1990) The role of alterations in membrane lipid composition in enabling physiological adaptation of organisms to their physical environment. Prog Lipid Res 29:167–227
Hsiao TY, Holmes B, Blanch HW (2007) Identification and functional analysis of a delta-6 desaturase from the marine microalga Glossomastix chrysoplasta. Mar Biotechnol 9:154–165
Huelsenbeck JP, Ronquist F (2001) MRBAYES: Bayesian inference of phylogenetic trees. Bioinformatics 17:754–755
Katoh K, Toh H (2008) Recent developments in the MAFFT multiple sequence alignment program. Brief Bioinform 9:286–298
Katoh K, Kuma K, Toh H, Miyata T (2005) MAFFT version 5: improvement in accuracy of multiple sequence alignment. Nucleic Acids Res 33:511–518
Kayukawa T, Chen B, Hoshizaki S, Ishikawa Y (2007) Upregulation of a desaturase is associated with the enhancement of cold hardiness in the onion maggot, Delia antiqua. Insect Biochem Mol Biol 37:1160–1167
Kostanjevečki V, Leys D, Van Driessche G, Meyer TE, Cusanovich MA, Fischer U, Guisez Y, Van Beeumen J (1999) Structure and characterization of Ectothiorhodospira vacuolata cytochrome b(558), a prokaryotic homologue of cytochrome b(5). J Biol Chem 274:35614–35620
Laoteng K, Mannontarat R, Tanticharoen M, Cheevadhanarak S (2000) Delta(6)-desaturase of Mucor rouxii with high similarity to plant delta(6)-desaturase and its heterologous expression in Saccharomyces cerevisiae. Biochem Biophys Res Commun 279:17–22
Libisch B, Michaelson LV, Lewis MJ, Shewry PR, Napier JA (2000) Chimeras of Delta6-fatty acid and Delta8-sphingolipid desaturases. Biochem Biophys Res Commun 279:779–785
López Alonso D, García-Maroto F, Rodríguez-Ruiz J, Garrido JA, Vilches MA (2003) Evolution of the membrane-bound fatty acid desaturases. Biochem Syst Ecol 31:1111–1124
Los DA, Murata N (1998) Structure and expression of fatty acid desaturases. Biochim Biophys Acta 1394:3–15
Lösel DM (1990) Lipids in the structure and function of fungal membranes. In: Kuhn PJ, Trinci APJ, Jung MJ, Goosey MW, Copping LG (eds) Biochemistry of the cell walls and membranes in fungi. Springer-Verlag, New York, pp 119–133
Meesapyodsuk D, Reed DW, Covello PS, Qiu X (2007) Primary structure, regioselectivity, and evolution of the membrane-bound fatty acid desaturases of Claviceps purpurea. J Biol Chem 282:20191–20199
Meesters PA, Springer J, Eggink G (1997) Cloning and expression of the delta 9 fatty acid desaturase gene from Cryptococcus curvatus ATCC 20509 containing histidine boxes and a cytochrome b5 domain. Appl Microbiol Biotechnol 47:663–667
Michaelson LV, Lazarus CM, Griffiths G, Napier JA, Stobart AK (1998) Isolation of a Delta5-fatty acid desaturase gene from Mortierella alpina. J Biol Chem 273:19055–19059
Michinaka Y, Aki T, Shimauchi T, Nakajima T, Kawamoto S, Shigeta S, Suzuki O, Ono K (2003) Differential response to low temperature of two Delta6 fatty acid desaturases from Mucor circinelloides. Appl Microbiol Biotechnol 62:362–368
Mitchell AG, Martin CE (1995) A novel cytochrome b5-like domain is linked to the carboxyl terminus of the Saccharomyces cerevisiae delta-9 fatty acid desaturase. J Biol Chem 270:29766–29772
Nakashima S, Zhao Y, Nozawa Y (1996) Molecular cloning of delta 9 fatty acid desaturase from the protozoan Tetrahymena thermophila and its mRNA expression during thermal membrane adaptation. Biochem J 317(Pt 1):29–34
Napier JA, Sayanova O, Stobart AK, Shewry PR (1997) A new class of cytochrome b5 fusion proteins. Biochem J 328(Pt 2):717–718
Napier JA, Sayanova O, Sperling P, Heinz E (1999) A growing family of cytochrome b5 fusion desaturases. Trends Plant Sci 4:2–5
Napier JA, Michaelson LV, Sayanova O (2003) The role of cytochrome b5 fusion desaturases in the synthesis of polyunsaturated fatty acids. Prostaglandins Leukot Essent Fatty Acids 68:135–143
Rilfors L, Lindblom G (2002) Regulation of lipid composition in biological membranes—biophysical studies of lipids and lipid synthesizing enzymes. Colloids Surf B Biointerfaces 26:112–124
Ronquist F, Huelsenbeck JP (2003) MrBayes 3: Bayesian phylogenetic inference under mixed models. Bioinformatics 19:1572–1574
Sakuradani E, Shimizu S (2009) Single cell oil production by Mortierella alpina. J Biotechnol 144:31–36
Schenkman JB, Jansson I (2003) The many roles of cytochrome b5. Pharmacol Ther 97:139–152
Shanklin J, Cahoon EB (1998) Desaturation and related modifications of fatty acids. Annu Rev Plant Physiol Plant Mol Biol 49:611–641
Shanklin J, Whittle E, Fox BG (1994) Eight histidine residues are catalytically essential in a membrane-associated iron enzyme, stearoyl-CoA desaturase, and are conserved in alkane hydroxylase and xylene monooxygenase. Biochemistry (Mosc) 33:12787–12794
Sperling P, Heinz E (2001) Desaturases fused to their electron donor. Eur J Lipid Sci Technol 103:158–180
Sperling P, Schmidt H, Heinz E (1995) A cytochrome-b5-containing fusion protein similar to plant acyl lipid desaturases. Eur J Biochem 232:798–805
Sperling P, Zahringer U, Heinz E (1998) A sphingolipid desaturase from higher plants. Identification of a new cytochrome b5 fusion protein. J Biol Chem 273:28590–28596
Sperling P, Ternes P, Zank TK, Heinz E (2003) The evolution of desaturases. Prostaglandins Leukot Essent Fatty Acids 68:73–95
Tamura K, Dudley J, Nei M, Kumar S (2007) MEGA4: molecular evolutionary genetics analysis (MEGA) software version 4.0. Mol Biol Evol 24:1596–1599
Thiede MA, Ozols J, Strittmatter P (1986) Construction and sequence of cDNA for rat liver stearyl coenzyme A desaturase. J Biol Chem 261:13230–13235
Yang Z (1997) PAML: a program package for phylogenetic analysis by maximum likelihood. Comput Appl Biosci 13:555–556
Yang Z (2007) PAML 4: phylogenetic analysis by maximum likelihood. Mol Biol Evol 24:1586–1591
Acknowledgements
We thank Prof. Aharon Oren from The Hebrew University of Jerusalem for critical reading of the manuscript and an anonymous reviewer of our previous paper (Gostinčar et al. 2009b) for focusing our attention on the questions regarding the cytochrome b5 domain of desaturases. This study was supported by the Ministry of Higher Education, Science and Technology of the Republic of Slovenia, in the form of a Young Researcher grant (to C. G.) and grant no. J1-6715.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Rights and permissions
About this article
Cite this article
Gostinčar, C., Turk, M. & Gunde-Cimerman, N. The Evolution of Fatty Acid Desaturases and Cytochrome b5 in Eukaryotes. J Membrane Biol 233, 63–72 (2010). https://doi.org/10.1007/s00232-010-9225-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-010-9225-x