Abstract
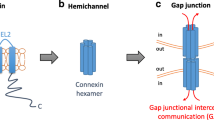
Gap junctions composed of connexin (Cx), a large protein family with a number of subtypes, are a main apparatus to maintain cellular homeostasis in many organs. Gap junctional intercellular communication (GJIC) is actively involved in all aspects of the cellular life cycle, ranging from cell growth to cell death. It is also known that the Cx gene acts as a tumor-suppressor due to the maintenance of cellular homeostasis via GJIC. In addition to this function, recent data show that the GJIC-independent function of Cx gene contributes to the tumor-suppressive effect of the gene with specificity to certain cells. With respect to the tumor-suppressive effects, Cx genes acts as tumor-suppressors in primary cancers, but the effects are still conflicting in invasive and metastatic cancers. We have previously reported that Cx32 is specifically downregulated in human renal cell carcinoma (RCC) cell lines as well as cancerous regions when compared to normal regions in kidneys. In recent studies, we have also reported that Cx32 suppresses growth, invasion and metastasis of RCC cells. In this minireview, we refer to a new aspect of Cx32-dependent functions against cell proliferation, invasion and metastasis in RCC cells, especially in a GJIC-independent manner.

Similar content being viewed by others
References
Ambudkar SV, Dey S, Hrycyna CA, Ramachandra M, Pastan I, Gottesman MM (1999) Biochemical, cellular, and pharmacological aspects of the multidrug transporter. Annu Rev Pharmacol Toxicol 39:361–398
Au JL, Jang SH, Zheng J, Chen CT, Song S, Hu L, Wientjes MG (2001) Determinants of drug delivery and transport to solid tumors. J Control Release 74:31–46
Bond SL, Bechberger JF, Khoo NK, Naus CC (1994) Transfection of C6 glioma cells with connexin32: the effects of expression of a nonendogenous gap junction protein. Cell Growth Differ 5:179–186
Byrne AM, Bouchier-Hayes DJ, Harmey JH (2005) Angiogenic and cell survival functions of vascular endothelial growth factor (VEGF). J Cell Mol Med 9:777–794
Dann CE 3rd, Bruick RK (2005) Dioxygenases as O2-dependent regulators of the hypoxic response pathway. Biochem Biophys Res Commun 338:639–647
Fitzgerald DJ, Yamasaki H (1990) Tumor promotion: models and assay systems. Teratog Carcinog Mutagen 10:89–102
Folkman J (1995) Seminars in medicine of the Beth Israel Hospital, Boston. Clinical applications of research on angiogenesis. N Engl J Med 333:1757–1763
Fujimoto E, Satoh H, Negishi E, Ueno K, Nagashima Y, Hagiwara K, Yamasaki H, Yano T (2004) Negative growth control of renal cell carcinoma cell by connexin 32: possible involvement of Her-2. Mol Carcinog 40:135–142
Fujimoto E, Sato H, Shirai S, Nagashima Y, Fukumoto K, Hagiwara H, Negishi E, Ueno K, Omori Y, Yamasaki H, Hagiwara K, Yano T (2005) Connexin32 as a tumor suppressor gene in a metastatic renal cell carcinoma cell line. Oncogene 24:3684–3690
Grunstein J, Roberts WG, Mathieu-Costello O, Hanahan D, Johnson RS (1999) Tumor-derived expression of vascular endothelial growth factor is a critical factor in tumor expansion and vascular function. Cancer Res 59:1592–1598
Hagiwara H, Sato H, Shirai S, Kobayashi S, Fukumoto K, Ishida T, Seki T, Ariga T, Yano T (2006) Connexin 32 down-regulates the fibrinolytic factors in metastatic renal cell carcinoma cells. Life Sci 78:2249–2254
Hirai A, Yano T, Nishikawa K, Suzuki K, Asano R, Satoh H, Hagiwara K, Yamasaki H (2003) Down-regulation of connexin 32 gene expression through DNA methylation in a human renal cell carcinoma cell. Am J Nephrol 23:172–177
Holder JW, Elmore E, Barrett JC (1993) Gap junction function and cancer. Cancer Res 53:3475–3485
Irby RB, Yeatman TJ (2000) Role of Src expression and activation in human cancer. Oncogene 19:5636–5642
Isaacs JS, Jung YJ, Mole DR, Lee S, Torres-Cabala C, Chung YL, Merino M, Trepel J, Zbar B, Toro J, Ratcliffe PJ, Linehan WM, Neckers L (2005) HIF overexpression correlates with biallelic loss of fumarate hydratase in renal cancer: novel role of fumarate in regulation of HIF stability. Cancer Cell 8:143–153
Jiang BH, Agani F, Passaniti A, Semenza GL (1997) V-SRC induces expression of hypoxia-inducible factor 1 (HIF-1) and transcription of genes encoding vascular endothelial growth factor and enolase 1: involvement of HIF-1 in tumor progression. Cancer Res 57:5328–5335
Kojima T, Sawada N, Chiba H, Kokai Y, Yamamoto M, Urban M, Lee GH, Hertzberg EL, Mochizuki Y, Spray DC (1999) Induction of tight junctions in human connexin32-transfected mouse hepatocytes: connexin32 interacts with occludin. Biochem Biophys Res Commun 266:222–229
Kojima T, Spray D, Kokai H, Chiba H, Mochizuki Y, Sawada N (2002) Cx32 formation and/or cx32-mediated intercellular communication induces expression and function of tight junctions in hepatocytic cell line. Exp Cell Res 276:40–51
Laird AD, Li G, Moss KG, Blake RA, Broome MA, Cherrington JM, Mendel DB (2003) Src family kinase activity is required for signal tranducer and activator of transcription 3 and focal adhesion kinase phosphorylation and vascular endothelial growth factor signaling in vivo and for anchorage-dependent and -independent growth of human tumor cells. Mol Cancer Ther 2:461–469
Li X, Kimura H, Hirota K, Kasuno K, Torii K, Okada T, Kurooka H, Yokota Y, Yoshida H (2005) Synergistic effect of hypoxia and TNF-alpha on production of PAI-1 in human proximal renal tubular cells. Kidney Int 68:569–583
Martyn KD, Kurata WE, Warn-Cramer BJ, Burt JM, Tenbroek E, Lau AF (1997) Immortalized connexin43 knockout cell lines display a subset of biological properties associated with the transformed phenotype. Cell Growth Differ 8:1015–1027
McMahon GA, Petitclere E, Stefansson S, Smith E, Wong MK, Westrick RJ, Ginsburg D, Brooks PC, Lawrence DA (2001) Plasminogen activator inhibitor-1 regulates tumor growth and angiogenesis. J Biol Chem 27:33964–33968
Mesnil M (2002) Connexins and cancer. Biol Cell 94:493–500
Mesnil M, Yamasaki H (1993) Cell-cell communication and growth control of normal and cancer cells: evidence and hypothesis. Mol Carcinog 7:14–17
Muthuswamy SK, Li D, Lelievre S, Bissell MJ, Brugge JS (2001) ErbB2, but not ErbB1, reinitiates proliferation and induces luminal repopulation in epithelial acini. Nat Cell Biol 3:785–792
Naito S, Sakamoto N, Kotoh S, Goto K, Matsumoto T, Kumazawa J (1993) Expression of P-glycoprotein and multidrug resistance in renal cell carcinoma. Eur Urol 24:156–160
Nicolson GL, Dulski KM, Trosko JE (1988) Loss of intercellular junctional communication correlates with metastatic potential in mammary adenocarcinoma cells. Proc Natl Acad Sci USA. 85:473–476
Paul DL (1995) New functions for gap junctions. Curr Opin Cell Biol 7:665–672
Paul R, Ewing CM, Robinson JC, Marshall FF, Johnson KR, Wheelock MJ, Isaacs WB (1997) Cadherin-6, a cell adhesion molecule specifically expressed in the proximal renal tubule and renal cell carcinoma. Cancer Res 57:2741–2748
Rae RS, Mehta PP, Chang CC, Trsoko JE, Ruch RJ (1998) Neoplastic phenotype of gap-junctional intercellular communication-deficient WB rat liver epithelial cells and its reversal by forced expression of connexin 32. Mol Carcinog 22:120–127
Ren Z, Schaefer TS (2002) ErbB-2 activates Stat3 alpha in a Src- and JAK2-dependent manner. J Biol Chem 277:38486–38493
Saez JC, Connor JA, Spray DC, Bennett MV (1989) Hepatocyte gap junctions are permeable to the second messenger, inositol 1,4,5-trisphosphate, and to calcium ions. Proc Natl Acad Sci USA 86:2708–2712
Sato H, Senba H, Virgona N, Fukumoto K, Ishida T, Hagiwara H, Negishi E, Ueno K, Yamasaki H, Yano T (2007) Connexin 32 potentiates vinblastine-induced cytotoxicity in renal cell carcinoma cells. Mol Carcinog 46:215–224
Selak MA, Armour SM, Mackenzie ED, Boulahbel H, Watson DG, Mansfield KD, Pan Y, Simon MC, Thompson CB, Gottlieb E (2005) Succinate links TCA cycle dysfunction to oncogenesis by inhibiting HIF-α prolyl hydroxylase. Cancer Cell 7:77–85
Semenza GL (1998) Hypoxia-inducible factor 1 and the molecular physiology of oxygen homeostasis. J Lab Clin Med 131:207–214
Simpson I, Rose B, Lowenstein WR (1977) Size limit of molecules permeating the junctional membrane channels. Science 195:294–296
Stauffer KA, Kumar NM, Gilula NB, Unwin N (1991) Isolation and purification of gap junction channels. J Cell Biol 115:141–150
Trosko JE, Madhukar BV, Chang CC (1993) Endogenous and exogenous modulation of gap junctional intercellular communication: toxicological and pharmacological implications. Life Sci 53:1–19
Trosko JE, Ruch RJ (2002) Gap junctions as targets for cancer chemoprevention and chemotherapy. Curr Drug Targets 3:465–482
Wartenberg M, Ling FC, Muschen M, Klein F, Acker H, Gassmann M, Petrat K, Putz V, Hescheler J, Sauer H (2003) Regulation of the multidrug resistance transporter P-glycoprotein in multicellular tumor spheroids by hypoxia-inducible factor (HIF-1) and reactive oxygen species. FASEB J 17:503–505
Willecke K, Eiberger J, Degen J, Eckardt D, Romualdi A, Guldenagel M, Deutsch U, Sohl G (2002) Structural and functional diversity of connexin genes in the mouse and human genome. Biol Chem 383:725–737
Yamasaki H, Naus CC (1996) Role of connexin genes in growth control. Carcinogenesis 17:1199–1213
Yano T (2007) A role of connexin 32 in regulation of metastasis of RCC cells. 2007 Annual Reports of Japan Human Health Foundations (in press)
Yano T, Hernandez-Blazquez FJ, Omori Y, Yamasaki H (2001) Reduction of malignant phenotype of HEPG2 cell is associated with the expression of connexin 26 but not connexin 32. Carcinogenesis 22:1593–1600
Yano T, Fujimoto E, Hagiwara H, Sato H, Yamasaki H, Negishi E, Ueno K (2006) Connexin 32 as an anti-invasive and anti-metastatic gene in renal cell carcinoma. Biol Pharm Bull 29:1991–1994
Yano T, Ito F, Satoh H, Hagiwara K, Nakazawa H, Toma H, Yamasaki H (2003) Tumor-suppressive effect of connexin 32 in renal cell carcinoma from maintenance hemodialysis patients. Kidney Int 63:381
Yonezawa Y, Nagashima Y, Sato H, Virgona N, Fukumoto K, Shirai S, Hagiwara H, Seki T, Ariga T, Senba H, Suzuki K, Asano R, Hagiwara K, Yano T (2005) Contribution of the Src family of kinases to the appearance of malignant phenotypes in renal cancer cells. Mol Carcinog 43:188–197
Acknowledgments
This study was supported by research grants for Health Sciences Focusing on Drug Innovation from the Japan Health Sciences Foundation (KH21012 and SH24209).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Sato, H., Hagiwara, H., Ohde, Y. et al. Regulation of Renal Cell Carcinoma Cell Proliferation, Invasion and Metastasis by Connexin 32 Gene. J Membrane Biol 216, 17–21 (2007). https://doi.org/10.1007/s00232-007-9020-5
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-007-9020-5