Abstract
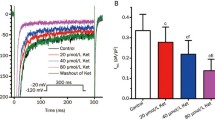
As an important in vivo antioxidant, vitamin C is commonly used clinically to alleviate hypoxia-induced heart symptoms. To approach the protective mechanisms of vitamin C on hearts during hypoxia, we investigated the electrophysiological effects of vitamin C (1 mM, pretreated before hypoxia) on Na+ currents (including transient and persistent Na+ currents) in guinea pig ventricular myocytes during hypoxia by the whole-cell and single-channel patch-clamp techniques. Whole-cell recordings showed that the mean current density of I NaT in the hypoxia group decreased from the control value of 40.2142 ± 1.7735 to 27.1663 ± 1.8441 pA/pF and current density of I NaP increased from 0.3987 ± 0.0474 to 1.1854 ± 01994 pA/pF (n = 9, P < 0.05 vs. control) at 15 min. However, when vitamin C was administered before hypoxia as pretreatment, I NaT and I NaP varied moderately (mean current density of I NaT decreasing from 41.6038 ± 2.9762 to 34.6341 ± 1.9651 pA/pF and current density of I NaP increasing from 0.3843 ± 0.0636 to 0.6734 ± 0.1057 pA/pF; n = 9, P < 0.05 vs. hypoxia group). Single-channel recordings (cell-patched) showed that the mean open probability and open time of I NaP increased significantly in both groups at hypoxia 15 min. However, the increased current values of the hypoxia group were still marked at hypoxia 15 min (n = 9, P < 0.05 vs. vitamin C + hypoxia group). Our results indicate that vitamin C can attenuate the disturbed effects of hypoxia on Na+ currents (I NaT and I NaP) of cardiac myocytes in guinea pigs effectively.




Similar content being viewed by others
References
Antzelevitch C. 2000. Electrical heterogeneity, cardiac arrhythmias, and the sodium channel. J. Circ. Res. 87:964–965
Bandyopadhyay D., Chattopadhyay A., Ghosh G., Datta A.G. 2004. Oxidative stress-induced ischemic heart disease: Protection by antioxidants. J. Curr. Med. Chem. 11:369–387
Barrington P.L., Martin R.L., Zhang K. 1997. Slowly inactivating sodium currents are reduced by exposure to oxidative stress. J. Mol. Cell. Cardiol. 29:3251–3265
Becker B.F., Kupatt C., Massoudy P., Zahler S. 2000. Reactive oxygen species and nitric oxide in myocardial ischemia and reperfusion. Z. Kardiol. 89 (Suppl. 9):IX/88–IX/91
Bielefeldt C.A., Whiteis M.W., Chapleau M.W., Abboud F.M. 1999. Nitric oxide enhances slow inactivation of voltage-dependent sodium currents in rat nodose neurons. Neurosci. Lett. 271:159–162
Bielefeldt K., Whiteis C.A., Sharma R.V., Abboud F.M., Conklin J.L. 1997. Reactive oxygen species and calcium homeostasis in intestinal smooth muscle cells. Am. J. Physiol. 272:G1439–G1450
Carr A.C.F.B. 1999. Vitamin C and cardiovascular disease. In: L. Cadenas, editor. Handbook of Antioxidants, 2nd ed., Marcel Dekker, Los Angeles pp. 147–165
Carr A.C., Frei B. 1999. Does vitamin C act as pro-oxidant under physiological conditions? The Federation of American Societies for Experimental Biology. FASEB J. 13:1007–1024
Cochrane, C. 1991. Cellular injury by oxidants. Am. J. Med. 91(Suppl. 3C):S23-S30
Daly, P., Sole, M.J. 1990. Myocardial catecholamines and the pathopysiology of heart failure. Circulation 82 (Suppl. l):1–35
Das D.K., Maulik N. 1994. Antioxidant effectiveness in ischemia-reperfusion tissue injury. J. Methods Enzymol. 233:601–610
Dhalla N.S., Temsah R.M., Netticadan T. 2000. Role of oxidative stress in cardiovascular diseases. J. Hypertens. 18:655–673
Duell, P. B. 1996. J. Nutr. 126:1067S
Duranteau J., Chandel N.S., Kulisz A., Shao Z., Schumacker P.T. 1998. Intracellular signaling by reactive oxygen species during hypoxia in cardiomyocytes. J. Biol. Chem. 273:11619–11624
Erbs S., Gielen S., Linke A., et al. 2003. Improvement of peripheral endothelial dysfunction by acute vitamin C application: Different effects in patients with coronary artery disease, ischemic, and dilated cardiomyopathy. Am. Heart J. 146:280–285
Ferrari R., Ceconi C., Curello S., et al. 1991. Oxygen free radicals and myocardial damage: Protective role of thiol-containing agents. Am. J. Med. 91:95S–105S
Gao F., Yao C.L., Gao E., et al. 2002. Enhancement of glutathione cardioprotection by ascorbic acid in myocardial reperfusion injury. J. Pharmacol. Exp. Ther. 301:543–550
Grech E.D., Jackson M., Ramsdale D.R. 1995. Reperfusion injury after acute myocardial infarction. Br. Med. J. 310:477–478
Guaiquil V.H., Golde D.W., Beckles D.L., Mascareno E.J., Siddiqui M.A.Q. 2004. Vitamin C inhibits hypoxia-induced damage and apoptotic signaling pathways in cardiomyocytes and ischemic hearts. J. Free Radic. Biol. Med. 37:1419–1429
Guaiquil V.H., Vera J.C., Golde D.W. 2001. Mechanism of vitamin C inhibition of cell death induced by oxidative stress in glutathione-depleted HL−60 cells. J. Biol. Chem. 276:40955–40961
Halliwell B.G.J. editor 1999. Free Radicals in Biology and Medicine, 3rd ed., Oxford University Press, Oxford
Hammarstrom A.K., Gage P.W. 1998. Inhibition of oxidative metabolism increases persistent sodium current in rat CA1 hippocampal neurons. J. Physiol. 510:735–741
Hammarstrom A.K., Gage P.W. 1999. Nitric oxide increases persistent sodium current in rat hippocampal neurons. J. Physiol. 520:451–461
Harsdorf R., Li P.F., Dietz R. 1999. Signaling pathways in reactive oxygen species-induced cardiomyocyte apoptosis. J. Circ. 99:2934–2941
Hayes N., McLellan L.I. 1999. Glutathione and glutathione-dependent enzymes represent a co-ordinately regulated defence against oxidative stress. J. Free Radic. Res. 31:273–300
Hensley K., Robinson K.A., Gabbita S.P., Salsman S., Floyd R.A. 2000. Reactive oxygen species, cell signaling, and cell injury. J. Free Radic. Biol. Med. 28:1456–1462
Ju Y.K., Saint D.A., Gage P.W. 1996. Hypoxia increases persistent sodium current in rat ventricular myocytes. J. Physiol. 497:337–347
Kitts, D. D. 1997. An evaluation of the multiple effects of the antioxidant vitamins. Trends Food Sci. Technol. 8:198–203
Kiyosue T., Arita M. 1989. Late sodium current and its contribution to action potential configuration in guinea pig ventricular myocytes. J. Circ Res. 64:389–397
Lefer A.M., Lefer D.J. 1993. Pharmacology of the endothelium in ischemia-reperfusion and circulatory shock. Annu. Rev. Pharmacol. Toxicol. 33:71–90
Lefer D.J., Granger D.N. 2000. Oxidative stress and cardiac disease. Am. J. Med. 109:315–323
Levi A.J., Dalton G.R., Hancox J.C., et al. 1997. Role of intracellular sodium overload in the genesis of cardiac arrhythmias. J. Cardiovasc. Electrophysiol. 8:700–721
Li Z., Chapleau M.W., Bates J.N., Bielefeidt K., Lee H.C., Abboud F.M. 1998. Nitric oxide as an autocrine regulator of sodium currents in baroreceptor neurons. J. Neuron 20:1039–1049
Luo A.T., Ma J.H., Zhang P.H. 2005. Effect of hydrogen peroxide on persistent sodium current and action potential in guinea pig ventricular myocytes. Acta Pharmacol. Sin. 26:769–896
Molyneux C.A., Glyn M.C., Ward B.J. 2002. Oxidative stress and cardiac microvascular structure in ischemia and reperfusion: The protective effect of antioxidant vitamins. J. Microvasc. Res. 64:265–277
Sakmann B.F., Spindler A.J., Bryant S.M., et al. 2000. Distribution of a persistent sodium current across the ventricular wall in guinea pigs. J. Circ Res. 87:910–914
Senthil S., Veerappan R.M., Ramakrishna Rao M., Pugalendi K.V. 2004. Oxidative stress and antioxidants in patients with cardiogenic shock complicating acute myocardial infarction. Clin. Chim. Acta 348:131–137
Stadtman, E.R. 1991. Ascorbic acid and oxidative inactiviation of proteins. Am. J. Clin. Nutr. 54:1125s
Sugiyama A., Hashimoto K. 1999. Can the MAP technique be applied to detect delayed after depolarization? Electrophysiologic and pharmacologic evidence. J. Cardiovasc. Pharmacol 34:46–52
Teramoto K., Daimon M., Hasegawa R., et al. 2004. Acute effect of oral vitamin C on coronary circulation in young healthy smokers. Am. Heart J. 148:300–305
Wang Q.D., Pernow J., Sjoquist P.O., Ryden L. 2002. Pharmacological possibilities for protection against myocardial reperfusion injury. J. Cardiovasc. Res. 55:25–37
Wang X.P., Ma J.H. 2004. Nitric oxide increases persistent sodium current of ventricular myocytes in guinea pig during normoxia and hypoxia. Acta Physiol. Sin. 56:603–608
Whalley D.W., Wendt D.J., Starmer C.F., et al. 1994. Voltage-independent effects of extracellular K+ on the Na+ current and phase 0 of the action potential in isolated cardiac myocytes. J. Circ Res. 75:491–502
Zabel M., Lichtlen P.R. 1998. Comparison of ECG variables of dispersion of ventricular repolarization with direct myocardial repolarization measurements in the human heart. J. Cardiovasc. Electrophysiol. 9:1279–1284
Zygmunt A.C., Eddlestone G.T., Thomas G.P., et al. 2001. Larger late sodium conductance in M cells contributes to electrical heterogeneity in canine ventricle. Am. J. Physiol. 281:H689–H697
Acknowledgement
This study was supported by grants from the Science Foundation of the Health Bureau of Hubei Province (JX2B72) and the Key Scientific Research Program of the Educational Bureau of Hubei Province (Z200511002).
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Zhou, H., Ma, JH., Zhang, PH. et al. Vitamin C Pretreatment Attenuates Hypoxia-Induced Disturbance of Sodium Currents in Guinea Pig Ventricular Myocytes. J Membrane Biol 211, 81–87 (2006). https://doi.org/10.1007/s00232-005-7014-8
Received:
Revised:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00232-005-7014-8