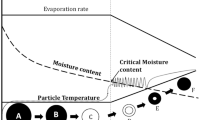
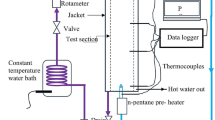
Abstract
This study examines the evaporative heat transfer and diffusive mass transfer of a droplet of CuCl2 solution. The validation of a new predictive model involves comparisons with experimental data from previous studies of different fluids based on non-dimensional analysis. The study provides new insight about the effects of different concentrations of water on the CuCl2 slurry drying at low to moderate air temperatures. Predictive correlations of heat and mass transfer are developed for the aqueous solution, subject to various drying conditions. The analysis is performed for moist air in contact with a sprayed aqueous solution of copper (II) chloride dihydrate [CuCl2·(2H2O)]. Results are presented and discussed for the drying processes.










Similar content being viewed by others
Abbreviations
- a w :
-
Activity of water (−)
- \(\overline{A}_{w}\) :
-
Partial molar surface area (m2)
- B :
-
Spalding number
- c i :
-
Molar concentration of ion (−)
- C p :
-
Heat capacity (kJ/kg K)
- d :
-
Diameter (m)
- \({\mathfrak{D}}\) :
-
Mass diffusion coefficient (m2/s)
- f s :
-
Enhancement factor (for ideal gas of water vapor) (−)
- g :
-
Acceleration due to gravity (m/s2)
- h :
-
External heat transfer coefficient (W/m2 K)
- HR :
-
Hausner ratio (−)
- ΔH v :
-
Latent heat of vaporization (kJ/kg)
- i:
-
Contributing ion
- k :
-
Coefficient of thermal conductivity (W/m K)
- \(\dot{m}\) :
-
Flow rate (kg/s)
- m :
-
Molecular weight (kg/mol)
- m l :
-
Molality (mol/kg)
- N :
-
Number of parameters
- p :
-
Pressure (Pa)
- p o :
-
Standard pressure (1,013.25 Pa)
- \(\dot{q}\) :
-
Heat flux density (W/m2)
- R :
-
Universal gas constant (kJ/kmol K); radius (m)
- RH :
-
Relative humidity (−)
- Re :
-
Reynolds number (−)
- T :
-
Temperature (°C) or (K)
- vf c :
-
Central void fraction (−)
- vf w :
-
Wall void fraction (−)
- V :
-
Volume, molecular volume (m3)
- v :
-
Volume fraction
- x :
-
Mass fraction (−)
- X :
-
Dry basis moisture content (kg H2O/kg dry substance)
- 0:
-
Initial, first
- 1:
-
Reference temperature, first
- 2:
-
Desired temperature, second
- 32:
-
Sauter mean
- a:
-
Air
- at.air :
-
Atomizing air
- aq:
-
Aqueous (in solution)
- av:
-
Average
- b:
-
Bulk gas, bulk fluid
- g:
-
Gas
- m:
-
Mass
- p :
-
Particle
- s:
-
Surface
- sat:
-
Saturation conditions
- sol:
-
Solution
- tot:
-
Total
- v:
-
Vapor
- w:
-
Water
- wb:
-
Wet bulb
- wv:
-
Water vapor
- θ :
-
Angle-direction
- α :
-
Contribution to thermal conductivity of ion (−), thermal diffusivity (m2/s)
- β :
-
Fitting parameters
- λ :
-
Constant
- μ :
-
Dynamic viscosity (Pa s)
- υ:
-
Kinematic viscosity (m2/s)
- ρ :
-
Density (kg/m3)
- σ :
-
Surface tension (N/m)
- ν :
-
Stoichiometric coefficient (−)
- θ :
-
Angle, surface-liquid contact angle (°)
- AECL:
-
Atomic Energy of Canada Limited
- CERL:
-
Clean Energy Research Laboratory
- DCC:
-
Copper chloride dihydrate
- GHG:
-
Greenhouse gas
- MSA:
-
Mean spherical approximation
- PCB:
-
Printed circuit board
- TC:
-
Thermochemical cycle
References
Naterer GF, Suppiah S, Stolberg L, Lewis M, Wang Z, Daggupati V, Gabriel K, Dincer I, Rosen MA, Spekkens P, Lvov SN, Fowler M, Tremaine P, Mostaghimi J, Easton EB, Trevani L, Rizvi G, Ikeda BM, Kaye MH, Lu L, Pioro I, Smith WR, Secnik E, Jiang J, Avsec J (2010) Canada’s program on nuclear hydrogen production and the thermochemical Cu–Cl cycle. Int J Hydrogen Energy 35:10905–10926
Lewis MA, Masin JG, O’Hare PA (2009) Evaluation of alternative thermochemical cycles, part I: the methodology. Int J Hydrogen Energy 34:4115–4124
Naterer GF, Suppiah S, Lewis M, Gabriel K, Dincer I, Rosen MA, Fowler M, Rizvi G, Easton E, Ikeda B (2009) Recent Canadian advances in nuclear-based hydrogen production and the thermochemical Cu–Cl cycle. Int J Hydrogen Energy 34:2901–2917
Suppiah S, Stolberg L, Boniface H, Tan G, McMahon S, York S, Zhang W (2009) Canadian nuclear hydrogen R&D programme: development of the medium-temperature Cu–Cl cycle and contributions to the high-temperature sulphur-iodine cycle. Fourth Information Exchange Meeting, Oakbrook, pp 14–16
Lewis MMA, Ferrandon MS, Tatterson DF, Mathias P (2009) Evaluation of alternative thermochemical cycles—part III further development of the Cu–Cl cycle. Int J Hydrogen Energy 34:4136–41455
Aghahosseini S, Dincer I, Naterer GF (2011) Integrated gasification and Cu–Cl cycle for trigeneration of hydrogen, steam and electricity. Int J Hydrogen Energy 36:2845–2854
Rosen MA, Naterer GF, Sadhankar R, Suppiah S (2012) Nuclear-based hydrogen production with a thermochemical copper-chlorine cycle and supercritical water reactor: equipment scale-up and process simulation. Int J Energy Res 36:456–465
Litwin RZ, Pinkowski SM (2008) Solar power for thermochemical production of hydrogen. US Patent Publication US 2008/0256952; Oct 23
Busscher N, Kahl J, Doesburg P, Mergardt G, Ploeger A (2010) Evaporation influences on the crystallization of an aqueous dihydrate cupric chloride solution with additives. J Colloid Interface Sci 344:556–562
Keskitalo T, Tanskanen J, Kuokkanen T (2007) Analysis of key patents of the regeneration of acidic cupric chloride etchant waste and tin stripping waste. Resour Conserv Recycl 49:217–243
Polyachenok OG, Dudkina EN, Branovitskaya NV, Polyachenok LD (2007) Formation of super disperse phase and its influence on equilibrium and thermodynamics of thermal dehydration. Thermochim Acta 467:44–53
Abdel Basir SM (2003) Recovery of cupric chloride from spent copper etchant solutions: a mechanistic study. Hydrometallurgy 69:135–143
Zamfirescu C, Dincer I, Naterer GF (2010) Thermophysical properties of copper compounds in copper–chlorine thermochemical water splitting cycles. Int J Hydrogen Energy 35:4839–4852
Mujumdar AS (2006) Handbook of industrial drying. CRC, Baco Raton
Daggupati VN, Naterer GF, Gabriel KS, Gravelsins RJ, Wang ZL (2011) Effects of atomization conditions and flow rates on spray drying for cupric chloride particle formation. Int J Hydrogen Energy 36:11353–11359
Ambike AA, Mahadik K, Paradkar A (2005) Spray-dried amorphous solid dispersions of simvastatin, a low Tg drug: in vitro and in vivo evaluations. Pharm Res 22:990–998
Ståhl K, Claesson M, Lilliehorn P, Lindén H, Bäckström K (2002) The effect of process variables on the degradation and physical properties of spray dried insulin intended for inhalation. Int J Pharm 233:227–237
Meenan P, Roberts K, Knight P, Yuregir K (1997) Influence of spray drying conditions on the particle properties of recrystallized burkeite (Na2CO3∙(Na2SO4)2). Powder Technol 90:125–130
Birchal VS, Passos ML, Wildhagen GRS, Mujumdar AS (2005) Effect of spray-dryer operating variables on the whole milk powder quality. Dry Technol 23:611–636
Jangam SV, Thorat BN (2010) Optimization of spray drying of ginger extract. Dry Technol 28:1426–1434
Xu X, Yao S, Han N, Shao B (2007) Measurement and influence factors of the flowability of microcapsules with high-content β-carotene. Chin J Chem Eng 15:579–585
Abdullah E, Geldart D (1999) The use of bulk density measurements as flowability indicators. Powder Technol 102:151–165
Orhan MF, Dincer I, Rosen MA (2011) Design of systems for hydrogen production based on the Cu–Cl thermochemical water decomposition cycle: configurations and performance. Int J Hydrogen Energy 36:11309–11320
Bharat V, Ray AK (1992) Evaporation and growth dynamics of a layered droplet. Int J Heat Mass Transf 35:2389–2401
Buyevich Y, Goldobin Y, Yasnikov GP (1994) Evolution of a particulate system governed by exchange with its environment. Int J Heat Mass Transf 37:3003–3014
Chen XD, Lin SXQ, Chen G (2002) On the ratio of heat to mass transfer coefficient for water evaporation and its impact upon drying modeling. Int J Heat Mass Transf 45:4369–4372
Saastamoinen JJ (2004) Heat exchange between two coupled moving beds by fluid flow. Int J Heat Mass Transf 47:1535–1547
Rasmussen K (1997) Calculation methods for the physical properties of air used in the calibration of microphones. Department of acoustic technology, Technical University of Denmark, Report PL-11b, Lyngby
Gilliland ER, Baddour RF, Perkinson GP, Sladek KJ (1974) Diffusion on surfaces. I. Effect of concentration on the diffusivity of physically adsorbed gases. Ind Eng Chem Fundam 13:95–100
Reid RC, Prausnitz JM, Poling BE (1987) The properties of gases and liquids. McGraw-Hill, New York
Incropera FP, Bergman TL, Lavine AS, DeWitt DP (2011) Fundamentals of heat and mass transfer. Wiley, New York
Laliberté M, Cooper WE (2004) Model for calculating the density of aqueous electrolyte solutions. J Chem Eng Data 49:1141–1151
Perry RH, Green DW (2008) Perry’s chemical engineers’ handbook. McGraw-Hill, New York
Laliberté M (2007) Model for calculating the viscosity of aqueous solutions. J Chem Eng Data 52:321–335
Riedel L (1951) Die wärmeleitfähigkeit von wäßrigen Lösungen starker elektrolyte. Chem Ing Tech 23:59–64
Wang P, Anderko A (2008) Modeling thermal conductivity of concentrated and mixed-solvent electrolyte systems. Ind Eng Chem Res 47:5698–5709
Goldberg RN (1979) Evaluated activity and osmotic coefficients for aqueous solutions: bi-univalent compounds of lead, copper, manganese and uranium. J Phys Chem Ref Data 8:1005–1050
Laliberté M (2009) A model for calculating the heat capacity of aqueous solutions, with updated density and viscosity data. J Phys Chem Ref Data 54:1725–1760
De Nevers N (2012) Physical and chemical equilibrium for chemical engineers. Wiley, New York
Polyachenok O, Dudkina E, Polyachenok L (2009) Thermal stability and thermodynamics of copper (II) chloride dihydrate. J Chem Thermodyn 41:74–79
Meaux L (1995) MaxRace Software & Meaux Racing Heads/Engines, Abbeville Los-Angeles
Babinsky E, Sojka PE (2002) Modeling drop size distributions. Prog Energy Combust Sci 28:303–329
Hede PD, Bach P, Jensen AD (2008) Two-fluid spray atomisation and pneumatic nozzles for fluid bed coating/agglomeration purposes: a review. Chem Eng Sci 63:3821–3842
Mulhem B, Schulte G, Fritsching U (2006) Solid-liquid separation in suspension atomization. Chem Eng Sci 61:2582–2589
Thybo P, Hovgaard L, Andersen SK, Lindeløv JS (2008) Droplet size measurements for spray dryer scale-up. Pharm Dev Technol 13:93–104
Sazhin SS (2006) Advanced models of fuel droplet heating and evaporation. Prog Energy Combust Sci 32:162–214
Acknowledgments
Financial support from Atomic Energy of Canada Limited and the Ontario Research Excellence Fund is gratefully acknowledged. The support of the members of the Clean Energy Research Laboratory (CERL) of University of Ontario Institute of Technology, Oshawa, ON, is also acknowledged.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Slowikowski, M., Naterer, G.F. & Odukoya, A. Diffusion properties of aqueous slurries in evaporative spray drying of copper (II) chloride dihydrate. Heat Mass Transfer 50, 1195–1210 (2014). https://doi.org/10.1007/s00231-014-1329-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00231-014-1329-x