Abstract.
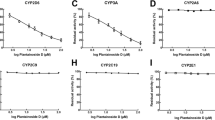
Objective: The aim of this investigation was to clarify the stereoselective properties in lansoprazole metabolism by monitoring the metabolic consumption for each enantiomer and the formation of the main metabolites, lansoprazole sulfone and 5-hydroxylansoprazole, in the presence of human liver microsomal enzymes. Methods: Human liver microsomes or recombinant cytochrome P 450 (CYP) enzymes were incubated with either (±)-, (+)-, or (–)-lansoprazole in the presence of reduced nicotinamide adenine dinucleotide phosphate. The metabolic consumption of lansoprazole enantiomers was estimated from the amounts of enantiomers consumed by microsomal enzymes after incubation at 37°C for 60 min. Metabolites of lansoprazole, lansoprazole sulfone, and 5-hydroxylansoprazole were determined after incubation at 37°C for 20 min, and kinetic parameters [Michaelis constant (K m) and maximum velocity (V max)] were obtained using Eadie-Hofstee plots. Results: (–)-Lansoprazole was metabolized more preferentially than (+)-lansoprazole in human liver microsomes. Stereoselective sulfoxidation [(–)>(+)] and hydroxylation [(+)>(–)] were observed in human liver microsomes. Strikingly, in sulfoxidation, a significantly higher intrinsic clearance (V max,1/K m,1) of (–)-lansoprazole (0.023±0.001 ml/min/mg) than (+)-lansoprazole (0.006±0.000 ml/min/mg) was observed. Consequently, sulfoxidation is likely to play an important role in the stereoselective metabolism of lansoprazole enantiomers. P 450-isoform specificity for each enantiomer was evident. CYP3A4, which mainly catalyzed sulfoxidation, was more active toward (–)-lansoprazole in either a chiral or racemic drug as a substrate. CYP2C19, which catalyzed hydroxylation, preferentially metabolized (+)-lansoprazole. The consumption of (+)-lansoprazole was markedly inhibited by (–)-lansoprazole, indicating a metabolic enantiomer/enantiomer interaction. However, this alteration of recombinant CYP2C19 specificity for (+)-lansoprazole did not appear in metabolism in human liver microsomes. Conclusions: Stereoselective metabolism was observed in human liver microsomes, and this stereoselectivity was mainly based on CYP3A4 specificity for preferable metabolism of (–)-lansoprazole.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Accepted in revised form: 23 August 2001
Electronic Publication
Rights and permissions
About this article
Cite this article
Katsuki, H., Hamada, A., Nakamura, C. et al. Role of CYP3A4 and CYP2C19 in the stereoselective metabolism of lansoprazole by human liver microsomes. Eur J Clin Pharmacol 57, 709–715 (2001). https://doi.org/10.1007/s002280100374
Received:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s002280100374