Abstract
Objective: The cytochrome P 450 isozymes CYP2D6 and CYP2C19 exhibit genetic polymorphism in human, including a marked interethnic difference. As the functional status of the isozymes CYP2D6 and CYP2C19 have an impact on the pharmacokinetics of some antidepressants, we investigated whether the disposition of venlafaxine was affected by the CYP2D6 and CYP2C19 genotypes.
Methods: Twenty-eight adult Japanese men in good health participated in this study. Genomic DNA was isolated from peripheral lymphocytes, and the CYP2D6 genotype was determined using polymerase chain reaction–restriction fragment length polymorphism (PCR-RFLP) analysis and XbaI-RFLP analysis. Subjects were categorized into the following four groups: group 1 CYP2D6*10/*10; group 2 CYP2D6*1/*10 and *2/*10; group 3 CYP2D6*1/*1, *1/*2 and *2/*2; and group 4 the other genotypes. Two defective CYP2C19 alleles (CYP2C19*2 and CYP2C19*3) were identified by means of PCR-RFLP analysis. Venlafaxine was administered orally following an overnight fast. Plasma concentrations of venlafaxine and O-desmethylvenlafaxine were monitored using high-performance liquid chromatography up to 24 h.
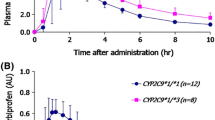
Results: The peak plasma concentration and values of area under the concentration–time curve up to 24 h for venlafaxine were 298% and 453% higher for group 1 than group 3, and 91% and 120% higher for group 2 than for group 3, respectively. The homozygote for two defective alleles of CYP2C19 showed a higher concentration of venlafaxine within group 1 and group 2.
Conclusion: The CYP2D6*10 allele and two CYP2C19 defective alleles, common in an Asian population, are the most likely genetic factors to use in determining interindividual differences in the pharmacokinetics of venlafaxine, although the results with respect to CYP2C19 are preliminary because of the few subjects used.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 6 July 1999 / Accepted in revised form 11 January 2000
Rights and permissions
About this article
Cite this article
Fukuda, T., Nishida, Y., Zhou, Q. et al. The impact of the CYP2D6 and CYP2C19 genotypes on venlafaxine pharmacokinetics in a Japanese population. E J Clin Pharmacol 56, 175–180 (2000). https://doi.org/10.1007/s002280050737
Issue Date:
DOI: https://doi.org/10.1007/s002280050737