Abstract
Objective: A single cross-over, comparative pharmacokinetic study of oral and rectal formulations of 200 mg artesunic acid in 12 healthy Malaysian volunteers is reported.
Methods: Plasma concentrations of artesunic acid and dihydroartemisinin were determined simultaneously by HPLC with electrochemical detection. The test drug was well tolerated and no undesirable adverse effects were observed.
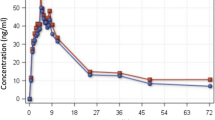
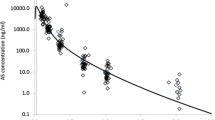
Results: Comparison of pharmacokinetic parameters of artesunic acid after oral and rectal administration showed statistically significant differences in t max and AUC, with no changes for C max and t 1/2. As for dihydroartemisinin, differences were observed for t max and C max but not for AUC.
Conclusion: There appear to be pharmacokinetic differences between oral and rectal modes of administration. The significance of these findings should be explored in malaria patients before appropriate therapeutic regimens are devised.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 14 January 1997 / Accepted in revised form: 24 February 1998
Rights and permissions
About this article
Cite this article
Navaratnam, V., Mansor, S., Mordi, M. et al. Comparative pharmacokinetic study of oral and rectal formulations of artesunic acid in healthy volunteers. E J Clin Pharmacol 54, 411–414 (1998). https://doi.org/10.1007/s002280050484
Issue Date:
DOI: https://doi.org/10.1007/s002280050484