Abstract
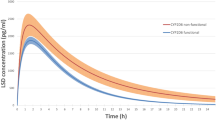
Objective: The purpose of this study was to investigate whether the disposition of fluvoxamine is associated with the CYP2D6 and CYP2C19 phenotype polymorphisms. Methods: The serum concentration of fluvoxamine was followed for 48 h after oral administration of a single dose of 50 mg fluvoxamine to five poor metabolizers of the CYP2D6 test drug dextromethorphan, five poor metabolizers of the CYP2C19 test drug mephenytoin, and five extensive metabolizers of both test drugs. Results: Poor metabolizers of dextromethorphan had significantly higher areas under the serum concentration-time curve than extensive metabolizers of dextromethorphan (mean 1.31 vs 1.00 μmol · h · l−1). There were no differences between poor and extensive metabolizers of mephenytoin (mean, 1.00 vs 1.15 μmol · h · l−1). Conclusion: The results are consistent with a possible minor to moderate role of CYP2D6, but not CYP2C19, in fluvoxamine metabolism.
Similar content being viewed by others
Author information
Authors and Affiliations
Additional information
Received: 25 April 1996 / Accepted in revised form: 12 November 1996
Rights and permissions
About this article
Cite this article
Spigset, O., Granberg, K., Hägg, S. et al. Relationship between fluvoxamine pharmacokinetics and CYP2D6/CYP2C19 phenotype polymorphisms. E J Clin Pharmacol 52, 129–133 (1997). https://doi.org/10.1007/s002280050261
Issue Date:
DOI: https://doi.org/10.1007/s002280050261