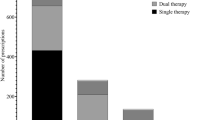
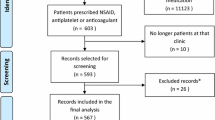
Abstract
Purpose
Due to potential drug-drug interactions and subsequent bleeding risk, analgesic use should be reviewed when an oral anticoagulant is initiated. The aim of this study was to compare use of non-steroidal anti-inflammatory drugs (NSAIDs) and other analgesics before and after oral anticoagulant initiation.
Methods
All individuals who initiated warfarin, dabigatran, or rivaroxaban between January 2012 and September 2013 were identified from the Finnish Prescription Register. Prevalence of analgesic use during 3 months after oral anticoagulant initiation was compared to analgesic use during 4 months before initiation. Analgesics included were NSAIDs, paracetamol, paracetamol in doses ≥2 g/day, tramadol, and other opioids. Conditional logistic regression was used to calculate odds ratios (OR) with 95 % confidence intervals (CI).
Results
In total, 54,025 initiated warfarin, 16,894 rivaroxaban, and 1569 dabigatran. The odds of NSAID use decreased among warfarin initiators (odds ratio (OR) 0.10; 95 % confidence interval (CI) 0.09–0.10); 2.6 % used NSAID after initiation. In contrast, the odds of NSAID use increased among rivaroxaban (OR 3.56; 95 % CI 3.37–3.75) and dabigatran initiators (OR 1.44; 95 % CI 1.16–1.78). The proportions using NSAIDs after the initiation were 69 and 32 %, respectively. However, NSAID use decreased among dabigatran initiators with confirmed atrial fibrillation (OR 0.46; 95 % CI 0.23–0.92) and among rivaroxaban initiators with a daily dose of ≥15 mg (OR 0.28; 95 % CI 0.19–0.40).
Conclusions
The use of NSAIDs decreases extensively among warfarin initiators which is encouraging. However, the use of NSAIDs increases among rivaroxaban and dabigatran initiators. This is a concern as the bleeding risk may increase due to potential pharmacodynamic interactions.





Similar content being viewed by others
References
Landefeld CS, Beyth RJ (1993) Anticoagulant-related bleeding: clinical epidemiology, prediction, and prevention. Am J Med 95:315–328
Antoniazzi S, Berdaï D, Conti V (2014) Risk of major bleeding and the standard doses of dabigatran. Eur J Intern Med 25:e73–e75
Huhtakangas J, Tetri S, Juvela S, Saloheimo P, Bode MK, Hillbom M (2011) Effect of increased warfarin use on warfarin-related cerebral hemorrhage: a longitudinal population-based study. Stroke 42:2431–2435
de Abajo FJ, Gil MJ, Bryant V, Timoner J, Oliva B, García-Rodríguez LA (2013) Upper gastrointestinal bleeding associated with NSAIDs, other drugs and interactions: a nested case–control study in a new general practice database. Eur J Clin Pharmacol 69:691–701
Hauta-Aho M, Tirkkonen T, Vahlberg T, Laine K (2009) The effect of drug interactions on bleeding risk associated with warfarin therapy in hospitalized patients. Ann Med 41:619–628
Schelleman H, Brensinger CM, Bilker WB, Hennessy S (2011) Antidepressant-warfarin interaction and associated gastrointestinal bleeding risk in a case–control study. PLoS One 6:e21447
Gagne JJ, Maio V, Rabinowitz C (2008) Prevalence and predictors of potential drug-drug interactions in Regione Emilia-Romagna, Italy. J Clin Pharm Ther 33:141–151
Malone D, Hutchins DS, Haupert H, Hansten P, Duncan B, Van Bergen RC, Solomon SL, Lipton RB (2005) Assessment of potential drug-drug interactions with a prescription claims database. Am J Health Syst Pharm 62:1983–1991
Roughead EE, Kalisch LM, Barratt JD, Gilbert AL (2010) Prevalence of potentially hazardous drug interactions amongst Australian veterans. Br J Clin Pharmacol 70:252–257
Cheetham TG, Levy G, Niu F, Bixler F (2009) Gastrointestinal safety of nonsteroidal antiinflammatory drugs and selective cyclooxygenase-2 inhibitors in patients on warfarin. Ann Pharmacother 43:1765–1773
Pottegård A, Meegaard PM, Holck LH, Christensen RD, Madsen H, Hallas J (2013) Concurrent use of tramadol and oral vitamin K antagonists and the risk of excessive anticoagulation: a register-based nested case–control study. Eur J Clin Pharmacol 69:641–646
Zhang Q, Bal-dit-Sollier C, Drouet L, Simoneau G, Alvarez J-C, Pruvot S, Aubourg R, Berge N, Bergmann J-F, Mouly S, Mahé I (2011) Interaction between acetaminophen and warfarin in adults receiving long-term oral anticoagulants: a randomized controlled trial. Eur J Clin Pharmacol 67:309–314
Hylek EM, Heiman H, Skates SJ, Sheehan MA, Singer DE (1998) Acetaminophen and other risk factors for excessive warfarin anticoagulation. JAMA 479:657–662
Schulman S (2014) Advantages and limitations of the new anticoagulants. J Intern Med 275:1–11
Cohen D (2014) Concerns over data in key dabigatran trial. Br Med J 349:g4747
Samoš M, Stančiaková L, Ivanková J, Staško J, Kovář F, Dobrotová M, Galajda P, Kubisz P, Mokáň M (2015) Monitoring of dabigatran therapy using Hemoclot Thrombin Inhibitor assay in patients with atrial fibrillation. J Thromb Thrombolysis 39:95–100
Chin PK, Patterson DM, Zhang M, Jensen BP, Wright DF, Barclay ML, Begg EJ (2014) Coagulation assays and plasma fibrinogen concentrations in real-world patients with atrial fibrillation treated with dabigatran. Br J Clin Pharmacol 78:630–638
Olesen JB, Sørensen R, Hansen ML, Lamberts M, Weeke P, Mikkelsen AP, Køber L, Gislason GH, Torp-Pedersen C, Fosbøl EL (2015) Non-vitamin K antagonist oral anticoagulation agents in anticoagulant naïve atrial fibrillation patients: Danish nationwide descriptive data 2011–2013. Europace 17:187–193
Savelieva I, Camm AJ (2014) Practical considerations for using novel oral anticoagulants in patients with atrial fibrillation. Clin Cardiol 37:32–47
Deftereos S, Anatoliotakis N, Giannopoulos G, Kaoukis A, Mavri M, Pyrgakis V, Stefanadis C (2012) Novel direct factor IIa and Xa inhibitors: mechanisms of action and preclinical studies. Curr Clin Pharmacol 7:149–165
Hellwig T, Gulseth M (2013) Pharmacokinetic and pharmacodynamic drug interactions with new oral anticoagulants: what do they mean for patients with atrial fibrillation? Ann Pharmacother 47:1478–1487
Reilly PA, Lehr T, Haertter S, Connolly SJ, Yusuf S, Eikelboom JW, Ezekowitz MD, Nehmiz G, Wang S, Wallentin L, Investigators RE-LY (2014) The effect of dabigatran plasma concentrations and patient characteristics on the frequency of ischemic stroke and major bleeding in atrial fibrillation patients: the RE-LY Trial (Randomized Evaluation of Long-Term Anticoagulation Therapy). J Am Coll Cardiol 63:321–328
Chin PK, Wright DF, Patterson DM, Doogue MP, Begg EJ (2014) A proposal for dose-adjustment of dabigatran etexilate in atrial fibrillation guided by thrombin time. Br J Clin Pharmacol 78:599–609
Wang Y, Bajorek B (2014) New oral anticoagulants in practice: pharmacological and practical considerations. Am J Cardiovasc Drugs 13:175–189
Masotti L, Lorenzini G, Seravalle C, Panigada G, Landini G, Cappelli R, Schulman S (2014) Management of new oral anticoagulants related life threatening or major bleedings in real life: a brief report. J Thromb Thrombolysis. doi:10.1007/s11239-014-1112-3
Vanden Daelen S, Peetermans M, Vanassche T, Verhamme P, Vandermeulen E (2015) Monitoring and reversal strategies for new oral anticoagulants. Expert Rev Cardiovasc Ther 13:95–103
Lindh JD, Andersson ML, Mannheimer B (2014) Adherence to guidelines for avoiding drug interactions associated with warfarin—a nationwide Swedish register study. PLoS One 9:e97388
Jobski K, Enders D, Amann U, Suzart K, Wallander MA, Schink T, Garbe E (2014) Use of rivaroxaban in Germany: a database drug utilization study of a drug started in hospital. Eur J Clin Pharmacol 70:975–981
Furu K, Wettermark B, Andersen M, Martikainen JE, Almarsdottir AB, Sorensen HT (2010) The Nordic countries as a cohort for pharmacoepidemiological research. Basic Clin Pharmacol Toxicol 106:86–94
WHO Collaborating Centre for Drug Statistics Methodology (2015) The anatomical therapeutic chemical classification system. Oslo: WHO Collaborating Centre for Drug Statistics Methodology. Norwegian Institute of Public Health. http://www.whocc.no/atcddd/. Accessed Jan 12, 2015
Social Insurance Institution of Finland (2014) Limited basic reimbursability for certain medicines. Social Insurance Institution of Finland. http://www.kela.fi/laakkeet-ja-laakekorvaukset_rajoitetun-korvattavuuden-alkamisajat. Accessed Oct 10, 2014
Trujillo T, Dobesh PP (2014) Clinical use of rivaroxaban: pharmacokinetic and pharmacodynamic rationale for dosing regimens in different indications. Drugs 74:1587–1603
Virjo I, Mäkelä K, Aho J, Kalliola P, Kurunmäki H, Uusitalo L, Valli M, Ylinen S (2010) Who receives anticoagulant treatment with warfarin and why? A population-based study in Finland. Scand J Prim Health Care 28:237–241
Finnish Medicines Agency Fimea, Social Insurance Institution of Finland (2013) Finnish statistics on medicines 2012. Fimea and Social Insurance Institution of Finland, Helsinki
Lanza FL, Chan FK, Quigley EM, Practice Parameters Committee of the American College of Gastroenterology (2009) Guidelines for prevention of NSAID-related ulcer complications. Am J Gastroenterol 104:728–738
Eriksson BI, Rosencher N, Friedman RJ, Homering M, Dahl OE (2012) Concomitant use of medication with antiplatelet effects in patients receiving either rivaroxaban or enoxaparin after total hip or knee arthroplasty. Thromb Res 130:147–151
Davidson BL, Verheijen S, Lensing AW, Gebel M, Brighton TA, Lyons RM, Rehm J, Prins MH (2014) Bleeding risk of patients with acute venous thromboembolism taking nonsteroidal anti-inflammatory drugs or aspirin. JAMA Intern Med 174:947–953
Friedman RJ, Kurth A, Clemens A, Noack H, Eriksson B, Caprini JA (2012) Dabigatran etexilate and concomitant use of non-steroidal antiinflammatory. Thromb Haemost 108:183–190
Kubitza D, Becka M, Mueck W, Zuehlsdorf M (2007) Rivaroxaban (BAY 59–7939)—an oral, direct Factor Xa inhibitor—has no clinically relevant interaction with naproxen. Br J Clin Pharmacol 63:469–476
Rachidi S, Aldin ES, Greenberg C, Sachs B, Streiff M, Zeidan AM (2013) The use of novel oral anticoagulants for thromboprophylaxis after elective major orthopedic surgery. Expert Rev Hematol 6:677–695
Working group appointed by the Finnish Medical Society Duodecim and the Finnish Orthopaedic Association (2011) Hip fracture: current care guideline. The Finnish Medical Society Duodecim, Helsinki
Working group appointed by the Finnish Medical Society Duodecim and the Finnish Orthopaedic Association (2012) Osteoarthritis: current care guideline. The Finnish Medical Society Duodecim, Helsinki
Koffeman AR, Valkhoff VE, Celik S, W’t Jong G, Sturkenboom MC, Bindels PJ, van der Lei J, Luijsterburg PA, Bierma-Zeinstra SM (2014) High-risk use of over-the-counter non-steroidal anti-inflammatory drugs: a population-based cross-sectional study. Br J Gen Pract 64:e191–e198
Acknowledgments
The authors would like to acknowledge Dr. Kris Jamsen at Monash University, Centre for Medicine Use and Safety, for his help producing the graphs.
Funding
We would like to acknowledge State funding for university-level health research (Grant L 3820 for Turku University Hospital) and the Social Insurance Institution of Finland (Grant 11/26/2012 for the University of Turku). Dr Jenni Ilomäki was supported by the National Health and Medical Research Council (Australia) fellowship.
Conflict of interest
The authors declare that they have no conflict of interest.
Author’s contributions
Jenni Ilomäki, Arja Helin-Salmivaara, Risto Huupponen, and Maarit Jaana Korhonen designed the study. Jenni Ilomäki, Maarit Jaana Korhonen, and Maria Rikala analyzed the data, and Jenni Ilomäki drafted the first version on the manuscript. All authors took part in interpretation of the data and gave critical comments to the manuscript. All authors approved the final version of the manuscript.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
ESM 1
(PDF 478 kb)
Rights and permissions
About this article
Cite this article
Ilomäki, J., Helin-Salmivaara, A., Huupponen, R. et al. Analgesic use before and after oral anticoagulant initiation—a population-based study in Finland. Eur J Clin Pharmacol 71, 723–732 (2015). https://doi.org/10.1007/s00228-015-1836-9
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-015-1836-9