Abstract
Purpose
This paper describes the pharmacokinetic/pharmacodynamic modelling of clazosentan in patients with aneurysmal subarachnoid haemorrhage (aSAH), and the impact of collecting data in an intensive care unit (ICU) setting. Factors influencing data quality, analysis, and interpretation are provided with recommendations for future clinical studies in ICU settings.
Methods
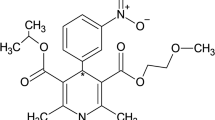
CONSCIOUS-2 was a phase III study involving 1,157 patients with aSAH. Secured by surgical clipping, patients were infused with clazosentan or placebo for up to 14 days post-aSAH. Clazosentan exposure relationships with vital signs, QT intervals, and AST/ALT values as well as efficacy and safety endpoints were characterised using population PK/PD and logistic regression models.
Results
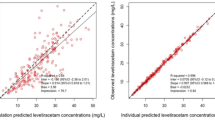
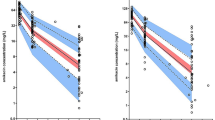
Clazosentan clearance was influenced by age, sex, Asian origin, and disease status at baseline, and increased with time. Volume of distribution showed a sex difference. Exposure had no relationship with any efficacy endpoint or ALT/AST values, but was related to the increasing probability of lung complications. Blood pressure decreased proportionally to clazosentan concentrations, and the presence of clazosentan was associated with QT interval increases. Implausible values in the concentration data reflect the specific ICU challenges, possibly arising from PK sampling from the infusion arm or haemodilution.
Conclusions
Population PK/PD modelling of CONCIOUS-2 data provided clinically relevant knowledge about various effects of clazosentan in the aSAH patient population in a real clinical setting. The quality of data and analyses could be improved by the collection of additional data and stricter training of study personnel. Differences in clinical practice between sites and geographical regions are more challenging to overcome.



Similar content being viewed by others
References
Hackett ML, Anderson CS (2000) Health outcomes 1 year after subarachnoid hemorrhage. An international population-based study. Neurology 55:658–662
Mayer SA, Kreiter KT, Copeland D et al (2002) Global and domain-specific cognitive impairment and outcome after subarachnoid hemorrhage. Neurology 59:1750–1758
Dorhout Mees SM, Rinkel GJ, Feigin (2007) Calcium antagonists for aneurysmal subarachnoid haemorrhage. Cochrane Database Syst Rev 3:CD000277
Egge A, Waterloo K, Sjoholm H, Solberg T, Ingebrigtsen T, Romner B (2001) Prophylactic hyperdynamic postoperative fluid therapy after aneurysmal subarachnoid hemorrhage: a clinical, prospective, randomized, controlled study. Neurosurgery 49:593–606
Rabinstein AA, Lanzino G, Wijdicks EF et al (2010) Multidisciplinary management and emerging therapeutic strategies in aneurysmal subarachnoid haemorrhage. Lancet Neurol 9:504–519
Gaab MR, Haubitz I, Brawanski A, Korn A, Czech T (1985) Acute effects of nimodipine on the cerebral blood flow and intracranial pressure. Neurochirurgia 28(Suppl 1):93–99
Pickard JD, Murray GD, Illingworth R et al (1990) Oral nimodipine and cerebral ischaemia following subarachnoid haemorrhage. Br J Clin Pract 44:66–67
Tettenborn D, Dycka J (1990) Prevention and treatment of delayed ischemic dysfunction in patients with aneurysmal subarachnoid hemorrhage. Stroke 21(12 Suppl):IV85–IV89
Macdonald RL, Pluta RM, Zhang JH (2007) Cerebral vasospasm after subarachnoid hemorrhage: the emerging revolution. Nat Clin Pract Neurol 3:256–263
Roux S, Löffler BM, Gray GA, Sprecher U, Clozel M, Clozel JP (1995) The role of endothelin in experimental cerebral vasospasm. Neurosurgery 37:78–85
van Giersbergen PL, Dingemanse J (2007) Tolerability, pharmacokinetics, and pharmacodynamics of clazosentan, a parenteral endothelin receptor antagonist. Eur J Clin Pharmacol 63:151–158
Bruderer S, Sasu B, Tsvitbaum N, Dingemanse J (2011) Influence of severe renal impairment on the pharmacokinetics of clazosentan. J Clin Pharmacol 51:413–421
Vajkoczy P, Meyer B, Weidauer S et al (2005) Clazosentan (AXV-034343), a selective endothelin a receptor antagonist, in the prevention of cerebral vasospasm following severe aneurysmal subarachnoid hemorrhage: results of a randomized, double-blind, placebo-controlled, multicenter phase IIa study. J Neurosurg 103:9–17
Macdonald RL, Kassell NF, Mayer S et al (2008) Clazosentan to overcome neurological ischemia and infarction occurring after subarachnoid hemorrhage (CONSCIOUS-1): randomized, double-blind, placebo-controlled phase 2 dose-finding trial. Stroke 39:3015–3021
Macdonald RL, Higashida RT, Keller E et al (2010) Preventing vasospasm improves outcome after aneurysmal subarachnoid hemorrhage: rationale and design of CONSCIOUS-2 and CONSCIOUS-3 trials. Neurocrit Care 13:416–424
Macdonald RL, Higashida RT, Keller E et al (2011) Clazosentan, an endothelin receptor antagonist, in patients with aneurysmal subarachnoid haemorrhage undergoing surgical clipping: a randomized, double-blind, placebo-controlled phase 3 trial (CONSCIOUS-2). Lancet Neurol 10:618–625
Teasdale G, Jennet B (1974) Assessment of coma and impaired consciousness. A practical scale. Lancet 2:81–84
Bruderer S, Detishin V, Tsvitbaum N, Dingemanse J (2011) Influence of different degrees of liver impairment on the pharmacokinetics of clazosentan. Br J Clin Pharmacol 71:52–60
Beal S, Sheiner LB, Boeckmann A, Bauer RJ (2009) NONMEM user’s guides (1989–2009). Icon Development Solutions, Ellicott City
Visual-NM Software, version 5.1 (1998) Montpellier, France: RDPP
SAS Software Package, version 8.2 (2001) Cary, NC: SAS Institute Inc
Sigma Plot, version 8.02 (2002) San Jose, CA: Systat Software Inc
Dingemanse J, Giersbergen PL (2004) Clinical pharmacology of bosentan, a dual endothelin receptor antagonist. Clin Pharmacokinet 43:1089–1115
Venitz J, Zack J, Gillies H, Allard M, Regnault J, Dufton C (2012) Clinical pharmacokinetics and drug-drug interactions of endothelin receptor antagonists in pulmonary arterial hypertension. J Clin Pharmacol 52:1784–1805
van Giersbergen PL, Treiber A, Dingemanse J (2009) In vitro and in vivo pharmacokinetic characteristics of clazosentan, an intravenous endothelin receptor antagonist, in humans. Int J Clin Pharmacol Ther 47:169–177
Benet LZ, Hoener BA (2002) Changes in plasma protein binding have little clinical relevance. Clin Pharm Ther 71:115–121
van Giersbergen PL, Gunawardena KA, Dingemanse J (2007) Influence of ethnic origin and sex on the pharmacokinetics of clazosentan. J Clin Pharmacol 47:1374–1380
van Giersbergen PL, Dingemanse J (2007) Effect of gender on the tolerability, safety and pharmacokinetics of clazosentan following long-term infusion. Clin Drug Investig 27:797–802
van Giersbergen PL, Dingemanse J (2008) Pharmacokinetic and pharmacodynamic aspects of the interaction between clazosentan and nimodipine in healthy subjects. Acta Neurochir Suppl 104:127–130
Sen J, Belli A, Albon H, Morgan L, Petzold A, Kitchen N (2003) Triple-H therapy in the management of aneurysmal subarachnoid haemorrhage. Lancet Neurol 2:614–621
Acknowledgments
Sébastien Roux (Actelion Pharmaceuticals Ltd) is acknowledged for the helpful discussions on clinical topics. Jürgen Karg at Inovalab, Reinach, Switzerland was responsible for the bioanalytical measurements.
Conflict of interest
Actelion Pharmaceuticals Ltd provided funding for this clinical trial and for the work conducted by Eliane Fuseau (EMF Consulting). Jochen Zisowsky, Shirin Bruderer, Andreas Krause, and Jasper Dingemanse are current employees and shareholders of Actelion Pharmaceuticals Ltd.
Author information
Authors and Affiliations
Corresponding author
Electronic supplementary material
Below is the link to the electronic supplementary material.
Online Resource 1
(PDF 30 kb)
Online Resource 2
(PDF 30 kb)
Online Resource 3
(PDF 33 kb)
Online Resource 4
(PDF 38 kb)
Online Resource 5
(PDF 575 kb)
Online Resource 6
(PDF 206 kb)
Online Resource 7
(PDF 98 kb)
Rights and permissions
About this article
Cite this article
Zisowsky, J., Fuseau, E., Bruderer, S. et al. Challenges in collecting pharmacokinetic and pharmacodynamic information in an intensive care setting: PK/PD modelling of clazosentan in patients with aneurysmal subarachnoid haemorrhage. Eur J Clin Pharmacol 70, 409–419 (2014). https://doi.org/10.1007/s00228-014-1647-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-014-1647-4