Abstract
Background
The management of schizophrenia has seen significant strides over the last few decades, due to the increasing availability of a number of antipsychotics. Yet, the diminished efficacy in relation to the negative and cognitive symptoms of schizophrenia, and the disturbing adverse reactions associated with the current antipsychotics, reflect the need for better molecules targeting unexplored pathways.
Purpose
To review the salient features of the recently approved antipsychotics; namely, iloperidone, asenapine, lurasidone and blonanserin.
Methods
We discuss the advantages, limitations and place in modern pharmacotherapy of each of these drugs. In addition, we briefly highlight the new targets that are being explored.
Results
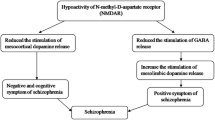
Promising strategies include modulation of the glutamatergic and GABAergic pathways, as well as cholinergic systems.
Conclusions
Although regulatory bodies have approved only a handful of antipsychotics in recent years, the wide spectrum of targets that are being explored could eventually bring out antipsychotics with improved efficacy and acceptability, as well as the potential to revolutionize psychiatric practice.
Similar content being viewed by others
Abbreviations
- BPRS:
-
Brief Psychiatric Rating Scale
- CANTAB:
-
Cambridge Neuropsychological Test Automated Battery
- CGI-S:
-
Clinical Global Impression Severity scale
- DLPFC:
-
Dorsolateral prefrontal cortex
- EPS:
-
Extrapyramidal symptoms
- MATRICS:
-
Measurement and Treatment Research to Improve Cognition in Schizophrenia
- MCCB:
-
Matrics Consensus Cognitive Battery
- nAchR:
-
Neuronal nicotinic acetylcholine receptors
- PANSS-T:
-
Positive and negative syndrome scale total score
- PDE:
-
Phosphodiesterase
- QoLS:
-
Quality of Life Scale
References
Kasckow J, Felmet K, Zisook S (2011) Managing suicide risk in patients with schizophrenia. CNS Drugs 25:129–143
Valenstein M, Blow FC, Copeland LA et al (2004) Poor antipsychotic adherence among patients with schizophrenia: medication and patient factors. Schizophr Bull 30:255–264
Purcell SM, Wray NR, Stone JL et al (2009) Common polygenic variation contributes to risk of schizophrenia and bipolar disorder. Nature 460:748–752
Albers LJ, Musenga A, Raggi MA (2008) Iloperidone: a new benzisoxazole atypical antipsychotic drug. Is it novel enough to impact the crowded atypical antipsychotic market? Expert Opin Investig Drugs 17:61–75
McGuire P, Howes OD, Stone J, Fusar-Poli P (2008) Functional neuroimaging in schizophrenia: diagnosis and drug discovery. Trends Pharmacol Sci 29:91–98
Potkin SG. PET Findings with Iloperidone. 2008. Available from: http://www.nccmedical.com/images/portfolio%20samples/USPMHC_Poster_2011.pdf Accessed on Feb 18, 2013
Arif SA, Mitchell MM (2011) Iloperidone: a new drug for the treatment of schizophrenia. Am J Health Syst Pharm 68:301–308
Rado J, Janicak PG (2010) Iloperidone for schizophrenia. Expert Opin Pharmacother 11:2087–2093
Subramanian N, Kalkman HO (2002) Receptor profile of P88-8991 and P95-12113, metabolites of the novel antipsychotic iloperidone. Prog Neuropsychopharmacol Biol Psychiatry 26:553–560
Hiemke C, Baumann P, Bergemann N et al (2011) AGNP consensus guidelines for therapeutic drug monitoring in psychiatry: update. Pharmacopsychiatry 44:195–235
Potkin SG, Litman RE, Torres R, Wolfgang CD (2008) Efficacy of iloperidone in the treatment of schizophrenia: initial phase 3 studies. J Clin Psychopharmacol 28:S4–S11
Cutler AJ, Kalali AH, Weiden PJ, Hamilton J, Wolfgang CD (2008) Four-week, double-blind, placebo- and ziprasidone-controlled trial of iloperidone in patients with acute exacerbations of schizophrenia. J Clin Psychopharmacol 28:S20–S28
Citrome L, Meng X, Hochfeld M, Stahl SM (2012) Efficacy of iloperidone in the short-term treatment of schizophrenia: a post hoc analysis of pooled patient data from four phase III, placebo- and active-controlled trials. Hum Psychopharmacol 27:24–32
Kane JM, Lauriello J, Laska E, Di Marino M, Wolfgang CD (2008) Long-term efficacy and safety of iloperidone: results from 3 clinical trials for the treatment of schizophrenia. J Clin Psychopharmacol 28:S29–S35
Weiden PJ, Cutler AJ, Polymeropoulos MH, Wolfgang CD (2008) Safety profile of iloperidone: a pooled analysis of 6-week acute-phase pivotal trials. J Clin Psychopharmacol 28:S12–S19
Vigneault P, Pilote S, Patoine D, Simard C, Drolet B (2012) Iloperidone fanapt(R), a novel atypical antipsychotic, is a potent HERG blocker and delays cardiac ventricular repolarization at clinically relevant concentration. Pharmacol Res. doi:10.1016/j.phrs.2012.03.008
Lavedan C, Licamele L, Volpi S et al (2009) Association of the NPAS3 gene and five other loci with response to the antipsychotic iloperidone identified in a whole genome association study. Mol Psychiatry 14:804–819
Volpi S, Potkin SG, Malhotra AK, Licamele L, Lavedan C (2009) Applicability of a genetic signature for enhanced iloperidone efficacy in the treatment of schizophrenia. J Clin Psychiatry 70:801–809
Volpi S, Heaton C, Mack K et al (2009) Whole genome association study identifies polymorphisms associated with QT prolongation during iloperidone treatment of schizophrenia. Mol Psychiatry 14:1024–1031
Minassian A, Young JW (2010) Evaluation of the clinical efficacy of asenapine in schizophrenia. Expert Opin Pharmacother 11:2107–2115
Shahid M, Walker GB, Zorn SH, Wong EH (2009) Asenapine: a novel psychopharmacologic agent with a unique human receptor signature. J Psychopharmacol 23:65–73
Bishara D, Taylor D (2009) Asenapine monotherapy in the acute treatment of both schizophrenia and bipolar I disorder. Neuropsychiatr Dis Treat 5:483–490
FDA Psychopharmacologic Drugs Advisory Committee Meeting (2009) Available from http://www.fda.gov/downloads/AdvisoryCommittees/CommitteesMeetingMaterials/Drugs/PsychopharmacologicDrugsAdvisoryCommittee/UCM173876.pdf Accessed on Feb 18, 2013
Citrome L (2011) Role of sublingual asenapine in treatment of schizophrenia. Neuropsychiatr Dis Treat 7:325–339
Weber J, McCormack PL (2009) Asenapine. CNS Drugs 23:781–792
Kane JM, Cohen M, Zhao J, Alphs L, Panagides J (2010) Efficacy and safety of asenapine in a placebo- and haloperidol-controlled trial in patients with acute exacerbation of schizophrenia. J Clin Psychopharmacol 30:106–115
Szegedi A, Verweij P, Van Dujinhoven W (2010) Eficacy of asenapine for schizophrenia: comparison with placebo and comparative efficacy of all atypical antipsychotics using all available head-to-head randomized trials using meta-analytical techniques. Neuropsychopharmacology 35:S105
Ishibashi T, Horisawa T, Tokuda K et al (2010) Pharmacological profile of lurasidone, a novel antipsychotic agent with potent 5-hydroxytryptamine 7 (5-HT7) and 5-HT1A receptor activity. J Pharmacol Exp Ther 334:171–181
Owen RT (2011) Lurasidone: a new treatment option for schizophrenia. Drugs Today (Barc) 47:807–816
Samalin L, Garnier M, Llorca PM (2011) Clinical potential of lurasidone in the management of schizophrenia. Ther Clin Risk Manag 7:239–250
Nakazawa S, Yokoyama C, Nishimura N et al (2013) Evaluation of dopamine D(2)/D(3) and serotonin 5-HT(2)a receptor occupancy for a novel antipsychotic, lurasidone, in conscious common marmosets using small-animal positron emission tomography. Psychopharmacology (Berl) 225:329–339
Meyer JM, Loebel AD, Schweizer E (2009) Lurasidone: a new drug in development for schizophrenia. Expert Opin Investig Drugs 18:1715–1726
Citrome L (2011) Lurasidone for schizophrenia: a brief review of a new second-generation antipsychotic. Clin Schizophr Relat Psychoses 4:251–257
Citrome L (2011) Lurasidone for schizophrenia: a review of the efficacy and safety profile for this newly approved second-generation antipsychotic. Int J Clin Pract 65:189–210
Harvey PD, Ogasa M, Cucchiaro J, Loebel A, Keefe RS (2011) Performance and interview-based assessments of cognitive change in a randomized, double-blind comparison of lurasidone vs. Ziprasidone. Schizophr Res 127:188–194
Ogasa MLACJ (2009) Effect of lurasidone on depressive symptoms in patients with schizophrenia. Schizophr Bull 35:344–345
Citrome L, Cucchiaro J, Sarma K et al (2012) Long-term safety and tolerability of lurasidone in schizophrenia: a 12-month, double-blind, active-controlled study. Int Clin Psychopharmacol 27:165–176
Lurasidone (Latuda) for schizophrenia (2011) Med Lett Drugs Ther 53:13–14
Citrome L (2011) Iloperidone, asenapine, and lurasidone: a brief overview of 3 new second-generation antipsychotics. Postgrad Med 123:153–162
Cruz MP (2011) Lurasidone HCl (latuda), an oral, once-daily atypical antipsychotic agent for the treatment of patients with schizophrenia. P T 36:489–492
Nakamura M, Ogasa M, Guarino J et al (2009) Lurasidone in the treatment of acute schizophrenia: a double-blind, placebo-controlled trial. J Clin Psychiatry 70:829–836
Potkin SG, Ogasa M, Cucchiaro J, Loebel A (2011) Double-blind comparison of the safety and efficacy of lurasidone and ziprasidone in clinically stable outpatients with schizophrenia or schizoaffective disorder. Schizophr Res 132:101–107
Deeks ED, Keating GM (2010) Blonanserin: a review of its use in the management of schizophrenia. CNS Drugs 24:65–84
Ohno Y, Okano M, Imaki J, Tatara A, Okumura T, Shimizu S (2010) Atypical antipsychotic properties of blonanserin, a novel dopamine D2 and 5-HT2A antagonist. Pharmacol Biochem Behav 96:175–180
Tateno A, Arakawa R, Okumura, M et al (2013) Striatal and Extrastriatal Dopamine D2 Receptor Occupancy by a Novel Antipsychotic, Blonanserin: A PET Study With [11C]Raclopride and [11C]FLB 457 in Schizophrenia. J Clin Psychopharmacol
Saruwatari J, Yasui-Furukori N, Inoue Y, Kaneko S (2010) Effect of dose timing in relation to food intake on systemic exposure to blonanserin. Eur J Clin Pharmacol 66:899–902
Garcia E, Robert M, Peris F, Nakamura H, Sato N, Terazawa Y (2009) The efficacy and safety of blonanserin compared with haloperidol in acute-phase schizophrenia: a randomized, double-blind, placebo-controlled, multicentre study. CNS Drugs 23:615–625
Yang J, Bahk WM, Cho HS et al (2010) Efficacy and tolerability of Blonanserin in the patients with schizophrenia: a randomized, double-blind, risperidone-compared trial. Clin Neuropharmacol 33:169–175
Tenjin T, Miyamoto S, Miyake N et al (2012) Effect of blonanserin on cognitive function in antipsychotic-naive first-episode schizophrenia. Hum Psychopharmacol 27:90–100
Kato K, Yamada K, Maehara M et al (2011) Blonanserin in the treatment of delirium. Psychiatry Clin Neurosci 65:389–391
Efficiency Study to Investigate Blonanserin in Treatment of Schizophrenia When Compared With Risperidone. Clinical Trials.Gov [updated 19 January 2012, cited 2012 Apr 14]. Available from [http://clinicaltrials.gov/ct2/show/NCT01516424?term=blonanserin&rank=1]
Kiss B, Horvath A, Nemethy Z et al (2010) Cariprazine (RGH-188), a dopamine D(3) receptor-preferring, D(3)/D(2) dopamine receptor antagonist-partial agonist antipsychotic candidate: in vitro and neurochemical profile. J Pharmacol Exp Ther 333:328–340
Seneca N, Finnema SJ, Laszlovszky I et al (2011) Occupancy of dopamine D2 and D3 and serotonin 5-HT1A receptors by the novel antipsychotic drug candidate, cariprazine (RGH-188), in monkey brain measured using positron emission tomography. Psychopharmacology (Berl) 218:579–587
Keator DB, Mukherjee J, Preda A (2009) Dopamine D2 and D3 receptor occupancy of cariprazine in schizophrenic patients. Schizophr Bull 35:154
Gyertyan I, Saghy K (2007) The selective dopamine D3 receptor antagonists, SB 277011-A and S 33084 block haloperidol-induced catalepsy in rats. Eur J Pharmacol 572:171–174
Citrome L. Cariprazine in Schizophrenia: Clinical Efficacy, Tolerability, and Place in Therapy. Adv Ther 2013 Jan 28
Patil ST, Zhang L, Martenyi F et al (2007) Activation of mGlu2/3 receptors as a new approach to treat schizophrenia: a randomized Phase 2 clinical trial. Nat Med 13:1102–1107
Kinon BJ, Zhang L, Millen BA et al (2011) A multicenter, inpatient, phase 2, double-blind, placebo-controlled dose-ranging study of LY2140023 monohydrate in patients with DSM-IV schizophrenia. J Clin Psychopharmacol 31:349–355
Liu F, Grauer S, Kelley C et al (2008) ADX47273 [S-(4-fluoro-phenyl)-{3-[3-(4-fluoro-phenyl)-[1, 2, 4]-oxadiazol-5-yl]-piperidin-1- yl}-methanone]: a novel metabotropic glutamate receptor 5-selective positive allosteric modulator with preclinical antipsychotic-like and procognitive activities. J Pharmacol Exp Ther 327:827–839
Buchanan RW, Keefe RS, Lieberman JA et al (2011) A randomized clinical trial of MK-0777 for the treatment of cognitive impairments in people with schizophrenia. Biol Psychiatry 69:442–449
Ochoa EL, Lasalde-Dominicci J (2007) Cognitive deficits in schizophrenia: focus on neuronal nicotinic acetylcholine receptors and smoking. Cell Mol Neurobiol 27:609–639
Sydserff S, Sutton EJ, Song D et al (2009) Selective alpha7 nicotinic receptor activation by AZD0328 enhances cortical dopamine release and improves learning and attentional processes. Biochem Pharmacol 78:880–888
Castner SA, Smagin GN, Piser TM et al (2011) Immediate and sustained improvements in working memory after selective stimulation of alpha7 nicotinic acetylcholine receptors. Biol Psychiatry 69:12–18
Freedman R, Olincy A, Buchanan RW et al (2008) Initial phase 2 trial of a nicotinic agonist in schizophrenia. Am J Psychiatry 165:1040–1047
Macor JE, Gurley D, Lanthorn T et al (2001) The 5-HT3 antagonist tropisetron (ICS 205-930) is a potent and selective alpha7 nicotinic receptor partial agonist. Bioorg Med Chem Lett 11:319–321
Cullum CM, Harris JG, Waldo MC et al (1993) Neurophysiological and neuropsychological evidence for attentional dysfunction in schizophrenia. Schizophr Res 10:131–141
Popkin MK (2008) Exacerbation of recurrent depression as a result of treatment with varenicline. Am J Psychiatry 165:774
Shiina A, Shirayama Y, Niitsu T et al (2010) A randomised, double-blind, placebo-controlled trial of tropisetron in patients with schizophrenia. Ann Gen Psychiatry 9:27
Shim JC, Jung DU, Jung SS et al (2012) Adjunctive varenicline treatment with antipsychotic medications for cognitive impairments in people with schizophrenia: a randomized double-blind placebo-controlled trial. Neuropsychopharmacology 37:660–668
Freedman R (2007) Exacerbation of schizophrenia by varenicline. Am J Psychiatry 164:1269
Tandon R, Nasrallah HA, Keshavan MS (2010) Schizophrenia, “just the facts” 5. Treatment and prevention. Past, present, and future. Schizophr Res 122:1–23
Ellenbroek BA (2012) Psychopharmacological treatment of schizophrenia: what do we have, and what could we get? Neuropharmacology 62:1371–1380
Abbott A (2011) Novartis to shut brain research facility. Nature 480:161–162
Lavedan C, Volpi S, Polymeropoulos MH, Wolfgang CD (2008) Effect of a ciliary neurotrophic factor polymorphism on schizophrenia symptom improvement in an iloperidone clinical trial. Pharmacogenomics 9:289–301
Food and Drug Administration. Drug approval package Fanapt (iloperidone) tablets: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/022192s007lbl.pdf; Accessed on Feb 18, 2013.
Food and Drug Administration. Drug approval package Saphris (asenapine) tablets: http://www.accessdata.fda.gov/drugsatfda_docs/label/2012/022117s013lbl.pdf; Accessed on Feb 18, 2013.
Food and Drug Administration. Drug approval package Latuda (lurasidone hydrochloride) tablets: http://www.accessdata.fda.gov/drugsatfda_docs/label/2013/200603s009s013lbl.pdf; Accessed on Feb 18, 2013.
Wen YG, Shang DW, Xie HZ et al (2013) Population pharmacokinetics of blonanserin in Chinese healthy volunteers and the effect of the food intake. Hum Psychopharmacol. doi:10.1002/hup.2290
A Study of LY2140023 in Patients with Schizophrenia. Clinical Trials.gov [updated 30 March 2012, cited 2012 Apr 14] Available from [http://clinicaltrials.gov/ct2/show/NCT01307800?term=NCT01307800&rank=1]
A Comparison Study of LY2140023 and Aripiprazole in Schizophrenia Patients. Clinical Trials.gov [updated 17 February 2012, cited 2012 Apr 14] Available from [http://clinicaltrials.gov/ct2/show/NCT01328093?term=NCT01328093&rank=1]
Schlumberger C, Pietraszek M, Gravius A, Danysz W (2010) Effects of a positive allosteric modulator of mGluR5 ADX47273 on conditioned avoidance response and PCP-induced hyperlocomotion in the rat as models for schizophrenia. Pharmacol Biochem Behav 95:23–30
de Lucena D, Fernandes BS, Berk M et al (2009) Improvement of negative and positive symptoms in treatment-refractory schizophrenia: a double-blind, randomized, placebo-controlled trial with memantine as add-on therapy to clozapine. J Clin Psychiatry 70:1416–1423
Lieberman JA, Papadakis K, Csernansky J et al (2009) A randomized, placebo-controlled study of memantine as adjunctive treatment in patients with schizophrenia. Neuropsychopharmacology 34:1322–1329
Hashimoto K (2011) Glycine transporter-1: a new potential therapeutic target for schizophrenia. Curr Pharm Des 17:112–120
Wezenberg E, Verkes RJ, Ruigt GS, Hulstijn W, Sabbe BG (2007) Acute effects of the ampakine farampator on memory and information processing in healthy elderly volunteers. Neuropsychopharmacology 32:1272–1283
Evaluation of Single and Repeat Doses of GSK729327 in Healthy Volunteers. Clinical Trials.gov [updated 14 October 2010, cited 2012 Apr 14] Available from [http://clinicaltrials.gov/ct2/show/NCT00448890?term=NCT00448890&rank=1]
Jardemark K, Marcus MM, Malmerfelt A, Shahid M, Svensson TH (2012) Differential effects of AMPA receptor potentiators and glycine reuptake inhibitors on antipsychotic efficacy and prefrontal glutamatergic transmission. Psychopharmacology (Berl) 221:115–131
Gamma-Amino Butyric Acid (GABA)-A Alpha2/3 Study. Clinical Trials.gov [updated 14 October 2011, cited 2012 Apr 14]Available from [http://clinicaltrials.gov/ct2/show/NCT00129441?term=NCT+00129441&rank=1]
Treating Schizophrenia by Correcting Abnormal Brain Development. Clinical Trials.gov [updated 28 July 2011, cited 2012 Apr 14]Available from [http://clinicaltrials.gov/ct2/show/NCT00179465?term=NCT00179465&rank=1]
A Study to Examine the Pharmacodynamic Effects of GSK1034702 on Neurophysiological Biomarkers of Cognition in Nicotine Abstained Otherwise Healthy Smokers (MAA113746). Clinical Trials.gov [updated 9 June 2011, cited 2012 Apr 14]Available from [http://clinicaltrials.gov/ct2/show/NCT01371799?term=NCT01371799&rank=1]
Bradley SR, Lameh J, Ohrmund L et al (2010) AC-260584, an orally bioavailable M(1) muscarinic receptor allosteric agonist, improves cognitive performance in an animal model. Neuropharmacology 58:365–373
Pharmacologic and Clinical Testing of a D1 Agonist for Cognitive Enhancement in Neuropsychiatric Disorders. Clinical Trials.gov [updated 24 January 2012, cited 2012 Apr 14]Available from [http://clinicaltrials.gov/ct2/show/NCT01519557?term=NCT01519557&rank=1]
Arbabi M, Bagheri M, Rezaei F et al (2012) A placebo-controlled study of the modafinil added to risperidone in chronic schizophrenia. Psychopharmacology (Berl) 220:591–598
Efficacy, Safety, and Tolerability of SPD489 in Adults with Schizophrenia and Predominant Negative Symptoms. Clinical Trials.gov [updated 2 February 2012, cited 2012 Apr 14] Available from [http://clinicaltrials.gov/ct2/show/NCT00922272?term=NCT00922272&rank=1]
Clinical Trial of Tolcapone for Cognition in Schizophrenia. Clinical Trials.gov [updated 20 March 2012, cited 2012 Apr 14] Available from [http://clinicaltrials.gov/ct2/show/NCT00044083?term=NCT00044083&rank=1]
Meltzer HY, Massey BW, Horiguchi M (2012) Serotonin Receptors as Targets for Drugs Useful to Treat Psychosis and Cognitive Impairment in Schizophrenia. Curr Pharm Biotechnol. Jan 26.[Abstract]
Safety and Cognitive Function Study of EVP-6124 in Patients with Schizophrenia. Clinical Trials.gov [updated 5 March 2012, cited 2012 April 14] Available from [http://clinicaltrials.gov/ct2/show/NCT00968851?term=NCT00968851&rank=1]
McLean SL, Idris N, Grayson B et al (2011) PNU-120596, a positive allosteric modulator of alpha7 nicotinic acetylcholine receptors, reverses a sub-chronic phencyclidine-induced cognitive deficit in the attentional set-shifting task in female rats. J Psychopharmacol. doi:10.1177/0269881111431747
Efficacy, Safety, and Tolerability of TC-5619 as Augmentation Therapy to Improve Negative Symptoms and Cognition in Outpatients with Schizophrenia. Clinical Trials.gov [updated 6 March 2012, cited 2012 April 14] . Available from [http://clinicaltrials.gov/ct2/show/NCT01488929?term=NCT01488929&rank=1]
Roncarati R, Scali C, Comery TA et al (2009) Procognitive and neuroprotective activity of a novel alpha7 nicotinic acetylcholine receptor agonist for treatment of neurodegenerative and cognitive disorders. J Pharmacol Exp Ther 329:459–468
Marcus MM, Wiker C, Franberg O et al (2010) Adjunctive alpha2-adrenoceptor blockade enhances the antipsychotic-like effect of risperidone and facilitates cortical dopaminergic and glutamatergic, NMDA receptor-mediated transmission. Int J Neuropsychopharmacol 13:891–903
Halene TB, Siegel SJ (2008) Antipsychotic-like properties of phosphodiesterase 4 inhibitors: evaluation of 4-(3-butoxy-4-methoxybenzyl)-2-imidazolidinone (RO-20-1724) with auditory event-related potentials and prepulse inhibition of startle. J Pharmacol Exp Ther 326:230–239
Yang SW, Smotryski J, McElroy WT et al (2012) Discovery of orally active pyrazoloquinolines as potent PDE10 inhibitors for the management of schizophrenia. Bioorg Med Chem Lett 22:235–239
Southam E, Cilia J, Gartlon JE et al (2009) Preclinical investigations into the antipsychotic potential of the novel histamine H3 receptor antagonist GSK207040. Psychopharmacology (Berl) 201:483–494
Yoshikawa S, Hareyama N, Ikeda K et al (2009) Effects of TRK-820, a selective kappa opioid receptor agonist, on rat schizophrenia models. Eur J Pharmacol 606:102–108
Levkovitz Y, Mendlovich S, Riwkes S et al (2010) A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J Clin Psychiatry 71:138–149
Muller N, Krause D, Dehning S et al (2010) Celecoxib treatment in an early stage of schizophrenia: results of a randomized, double-blind, placebo-controlled trial of celecoxib augmentation of amisulpride treatment. Schizophr Res 121:118–124
Pedersen CA, Gibson CM, Rau SW et al (2011) Intranasal oxytocin reduces psychotic symptoms and improves Theory of Mind and social perception in schizophrenia. Schizophr Res 132:50–53
Javitt DC, Buchanan RW, Keefe RS et al (2012) Effect of the neuroprotective peptide davunetide (AL-108) on cognition and functional capacity in schizophrenia. Schizophr Res 136:25–31
Study of Talnetant versus Placebo and Risperidone In Schizophrenia. Clinical Trials.gov [updated 1 March 2012, cited 2012 April 14] Available from [http://clinicaltrials.gov/ct2/show/NCT00103727?term=NCT00103727&rank=1]
Weiser M, Gershon AA, Rubinstein K et al (2012) A randomized controlled trial of allopurinol vs. placebo added on to antipsychotics in patients with schizophrenia or schizoaffective disorder. Schizophr Res 136:25–31
Rasagiline in the Treatment of Persistent Negative Symptoms of Schizophrenia. Clinical Trials.gov [updated 23 April 2011, cited 2012 April 14] Available from [http://clinicaltrials.gov/ct2/show/NCT00492336?term=NCT00492336&rank=1]
Bexarotene Augmentation of Antipsychotic Treatment for Chronic Schizophrenia. Clinical Trials.gov [updated 28 June 2010, cited 2012 April 14]. Available from [http://clinicaltrials.gov/ct2/show?term=NCT00141947&rank=2]
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
George, M., Amrutheshwar, R., Rajkumar, R.P. et al. Newer antipsychotics and upcoming molecules for schizophrenia. Eur J Clin Pharmacol 69, 1497–1509 (2013). https://doi.org/10.1007/s00228-013-1498-4
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-013-1498-4