Abstract
Purpose
The pharmacokinetics (PK) of labetalol show wide inter-subject variability, but the genetic causes for this are largely undetermined. This study was performed to examine whether common polymorphisms in UGT1A1, UGT2B7, CYP2C19 and ABCB1 affect the PK of labetalol.
Methods
The PK of labetalol were determined in 37 Chinese healthy male subjects who took a single oral dose of 200 mg labetalol. Plasma concentrations of labetalol were determined by a high-performance liquid chromatographic method. Subjects were genotyped for the CYP2C19*2 and *3, UGT1A1 *6, *28 and *60, UGT2B7*2 and ABCB1 1236C>T, 2677G>T/A and 3435C>T polymorphisms.
Results
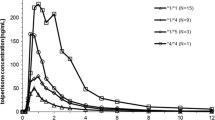
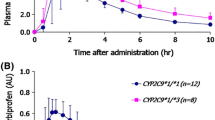
Subjects with the CYP2C19*2/*2 genotype had a higher peak concentration (255.5 ± 80.1 vs. 156.0 ± 66.3 ng/mL; P < 0.05) and area under the concentration–time curve (AUC0−∞; 1,473.7 ± 493.6 vs. 502.8 ± 176.1 ng⋅h/mL; P < 0.001) than subjects with the *1/*1 genotype, and heterozygotes had intermediate values. The common UGT polymorphisms, UGT1A1*6, *60 or *28, and UGT2B7*2 did not result in a significant effect. Subjects with ABCB1 2677TA or TT or ABCB1 3435TT genotypes had higher AUC0−∞ and lower total clearance than the wild-types (P < 0.05), but this appeared to be related to the distribution of CYP2C19 genotypes. The CYP2C19 genotype appeared to be the only predictor of labetalol concentrations, accounting for approximately 60 % of the total variance in the AUC0−∞.
Conclusion
Our results suggest that the PK of labetalol are significantly affected by the common CYP2C19 polymorphisms in individuals of Chinese ethnicity. Future larger studies are needed to evaluate the effect of CYP2C19 and UGT1A1 polymorphisms on the PK of labetalol stereoisomers and the pharmacodynamic effects.



Similar content being viewed by others
References
Richards DA, Prichard BN (1979) Clinical pharmacology of labetalol. Br J Clin Pharmacol 8[Suppl 2]:89S–93S
Frishman WH, Saunders E (2011) Beta-adrenergic blockers. J Clin Hypertens 13(9):649–653
Goa KL, Benfield P, Sorkin EM (1989) Labetalol—a reappraisal of its pharmacology, pharmacokinetics and therapeutic use in hypertension and ischemic heart-disease. Drugs 37(5):583–627
Mcneil JJ, Louis WJ (1984) Clinical pharmacokinetics of labetalol. Clin Pharmacokinet 9(2):157–167
Jeong H, Choi S, Song JW, Chen H, Fischer JH (2008) Regulation of UDP-glucuronosyltransferase (UGT) 1A1 by progesterone and its impact on labetalol elimination. Xenobiotica 38(1):62–75
Carvalho TM, Cavalli Rde C, Cunha SP, de Baraldi CO, Marques MP, Antunes NJ, Godoy AL, Lanchote VL (2011) Influence of gestational diabetes mellitus on the stereoselective kinetic disposition and metabolism of labetalol in hypertensive patients. Eur J Clin Pharmacol 67(1):55–61
Carvalho TM, Cavalli Rde C, Marques MP, Da Cunha SP, Baraldi Cde O, Lanchote VL (2009) Stereoselective analysis of labetalol in human plasma by LC-MS/MS: application to pharmacokinetics. Chirality 21(8):738–744
Johnson JA, Akers WS, Herring VL, Wolfe MS, Sullivan JM (2000) Gender differences in labetalol kinetics: importance of determining stereoisomer kinetics for racemic drugs. Pharmacotherapy 20(6):622–628
Lalonde RL, O’Rear TL, Wainer IW, Drda KD, Herring VL, Bottorff MB (1990) Labetalol pharmacokinetics and pharmacodynamics: evidence of stereoselective disposition. Clin Pharmacol Ther 48(5):509–519
Tenero DM, Bottorff MB, Given BD, Kramer WG, Affrime MB, Patrick JE, Lalonde RL (1989) Pharmacokinetics and pharmacodynamics of dilevalol. Clin Pharmacol Ther 46(6):648–656
Gal J, Zirrolli JA, Lichtenstein PS (1988) Labetalol is metabolized oxidatively in humans. Res Commun Chem Pathol Pharmacol 62(1):3–17
Martin LE, Hopkins R, Bland R (1976) Metabolism of labetalol by animals and man. Br J Clin Pharmacol 3[4 Suppl 3]:695–710
Daneshmend TK, Roberts CJC (1984) The effects of enzyme-induction and enzyme-inhibition on labetalol pharmacokinetics. Br J Clin Pharmacol 18(3):393–400
Abushammala I, Garrigues TM, Casabo VG, Nacher A, Martin-Villodre A (2006) Labetalol absorption kinetics: rat small intestine and colon studies. J Pharm Sci 95(8):1733–1741
Christian H, Bertera FM, Mayer MA, Taira CA (2010) Issues in drug metabolism of major antihypertensive drugs: beta-blockers, calcium channel antagonists and angiotensin receptor blockers. Expert Opin Drug Metab Toxicol 6(2):199–211
Zhou Q, Yu XM, Lin HB, Wang L, Yun QZ, Hu SN, Wang DM (2009) Genetic polymorphism, linkage disequilibrium, haplotype structure and novel allele analysis of CYP2C19 and CYP2D6 in Han Chinese. Pharmacogenomics J 9(6):380–394
Ando Y, Fujita K, Sasaki Y, Hasegawa Y (2007) UGT1AI*6 and UGT1A1*27 for individualized irinotecan chemotherapy. Curr Opin Mol Ther 9(3):258–262
Zhang X, Meng X, Wang Y, Yan W, Yang J (2012) Comprehensive analysis of UGT1A1 genetic polymorphisms in Chinese Tibetan and Han populations. Biochem Genet [Epub ahead of print]
Fung KL, Gottesman MM (2009) A synonymous polymorphism in a common MDR1 (ABCB1) haplotype shapes protein function. Biochim Biophys Acta 1794(5):860–871
Kaniwa N, Kurose K, Jinno H, Tanaka-Kagawa T, Saito Y, Saeki M, Sawada J, Tohkin M, Hasegawa R (2005) Racial variability in haplotype frequencies of UGT1A1 and glucuronidation activity of a novel single nucleotide polymorphism 686C > T (P229L) found in an African-American. Drug Metab Dispos 33(3):458–465
Court MH, Krishnaswamy S, Hao Q, Duan SX, Patten CJ, Von Moltke LL, Greenblatt DJ (2003) Evaluation of 3′-azido-3′-deoxythymidine, morphine, and codeine as probe substrates for UDP-glucuronosyltransferase 2B7 (UGT2B7) in human liver microsomes: specificity and influence of the UGT2B7*2 polymorphism. Drug Metab Dispos 31(9):1125–1133
Bhasker CR, McKinnon W, Stone A, Lo ACT, Kubota T, Ishizaki T, Miners JO (2000) Genetic polymorphism of UDP-glucuronosyltransferase 2B7 (UGT2B7) at amino acid 268: ethnic diversity of alleles and potential clinical significance. Pharmacogenetics 10(8):679–685
Girard C, Barbier O, Veilleux G, El-Alfy M, Belanger A (2003) Human uridine diphosphate-glucuronosyltransferase UGT2B7 conjugates mineralocorticoid and glucocorticoid metabolites. Endocrinology 144(6):2659–2668
Lin JP, O’Donnell CJ, Schwaiger JP, Cupples LA, Lingenhel A, Hunt SC, Yang S, Kronenberg F (2006) Association between the UGT1A1*28 allele, bilirubin levels, and coronary heart disease in the Framingham Heart Study. Circulation 114(14):1476–1481
Rodrigues C, Vieira E, Santos R, de Carvalho J, Santos-Silva A, Costa E, Bronze-da-Rocha E (2012) Impact of UGT1A1 gene variants on total bilirubin levels in Gilbert syndrome patients and in healthy subjects. Blood Cells Mol Dis 48(3):166–172
Minami H, Sai K, Saeki M, Saito Y, Ozawa S, Suzuki K, Kaniwa N, Sawada JI, Hamaguchi T, Yamamoto N, Shirao K, Yamada Y, Ohmatsu H, Kubota K, Yoshida T, Ohtsu A, Saijo N (2007) Irinotecan pharmacokinetics/pharmacodynamics and UGT1A genetic polymorphisms in Japanese: roles of UGT1A1*6 and *28. Pharmacogenet Genomics 17(7):497–504
Yamamoto N, Takahashi T, Kunikane H, Masuda N, Eguchi K, Shibuya M, Takeda Y, Isobe H, Ogura T, Yokoyama A, Watanabe K (2009) Phase I/II pharmacokinetic and pharmacogenomic study of UGT1A1 polymorphism in elderly patients with advanced non-small cell lung cancer treated with irinotecan. Clin Pharmacol Ther 85(2):149–154
Bohmer GM, Gleiter CH, Morike K, Nassr N, Walz A, Lahu G (2011) No dose adjustment on coadministration of the PDE4 inhibitor roflumilast with a weak CYP3A, CYP1A2, and CYP2C19 inhibitor: an investigation using cimetidine. J Clin Pharmacol 51(4):594–602
Garcia-Barcelo M, Chow LY, Chiu HFK, Wing YK, Lee DTS, Lam KL, Waye MMY (1999) Frequencies of defective CYP2C19 alleles in a Hong Kong Chinese population: detection of the rare allele CYP2C19*4. Clin Chem 45(12):2273–2274
Bogman K, Silkey M, Chan SP, Tomlinson B, Weber C (2010) Influence of CYP2C19 genotype on the pharmacokinetics of R483, a CYP2C19 substrate, in healthy subjects and type 2 diabetes patients. Eur J Clin Pharmacol 66(10):1005–1015
Hodges LM, Markova SM, Chinn LW, Gow JM, Kroetz DL, Klein TE, Altman RB (2011) Very important pharmacogene summary: ABCB1 (MDR1, P-glycoprotein). Pharmacogenet Genomics 21(3):152–161
Siegmund W, Ludwig K, Giessmann T, Dazert P, Schroeder E, Sperker B, Warzok R, Kroemer HK, Cascorbi I (2002) The effects of the human MDR1 genotype on the expression of duodenal P-glycoprotein and disposition of the probe drug talinolol. Clin Pharmacol Ther 72(5):572–583
Acknowledgments
We would like to thank the study volunteers for their participation and the other members of the research team who contributed to this study, especially Ms. Evelyn Chau and Ms. Swen Ip for the clinical study, Ms. Emily Poon for the genotyping and Ms. Sherry S. L. Lam for the drug assay.
Conflicts of interest/disclosure
The bioequivalence studies were supported by Vickmans Laboratories Limited, Hong Kong. The authors have no potential conflicts of interest with respect to this research.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Chan, S.W., Hu, M., Ko, S.S.W. et al. CYP2C19 genotype has a major influence on labetalol pharmacokinetics in healthy male Chinese subjects. Eur J Clin Pharmacol 69, 799–806 (2013). https://doi.org/10.1007/s00228-012-1428-x
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-012-1428-x