Abstract
Purpose
To determine whether hepatic impairment has an effect on the pharmacokinetics (PK) of vorapaxar or M20, its main pharmacologically active metabolite.
Methods
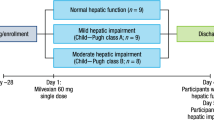
This was an open-label study in which a single 40-mg oral dose of vorapaxar was administered to patients with mild (n = 6), moderate (n = 6), and severe (n = 4) hepatic impairment and healthy controls (n = 16) matched for age, gender, weight, and height. Blood samples for vorapaxar and M20 assay were collected predose and at frequent intervals up to 8 weeks postdose.
Results
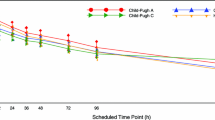
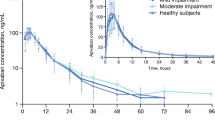
Plasma vorapaxar and M20 PK profiles were similar between patients with impaired liver function and healthy controls. Group mean values for vorapaxar Cmax and AUCtf were 206–279 ng/mL and 14,200–18,200 ng·h/mL, respectively, with the lowest values observed in patients with severe impairment. Vorapaxar median Tmax and mean t1/2 values were 1.00–1.75 h and 298–366 h, respectively. There was no apparent correlation between vorapaxar or M20 exposure or t1/2 values and disease severity. Vorapaxar was generally well tolerated; one serious adverse event (gastrointestinal bleeding secondary to ruptured esophageal varices) was reported in a patient with severe hepatic impairment.
Conclusions
Hepatic impairment had no clinically relevant effect on the PK of vorapaxar and M20. No dose or dosage adjustment of vorapaxar will be required in patients with mild to moderate hepatic impairment. Although systemic exposure to vorapaxar does not appear to increase in patients with severe hepatic impairment, administration of vorapaxar to such patients is not recommended given their bleeding diathesis.

Similar content being viewed by others
References
Antiplatelet Trialists’ Collaboration (1994) Collaborative overview of randomised trials of antiplatelet therapy—I: prevention of death, myocardial infarction, and stroke by prolonged antiplatelet therapy in various categories of patients. Br Med J 308:81–106
CAPRIE Steering Committee (1996) A randomised, blinded trial of clopidogrel versus aspirin in patients at risk of ischaemic events (CAPRIE). Lancet 348:1329–1339
Dogne JM, de Leval X, Benoit P, Delarge J, Masereel B, David JL (2002) Recent advances in antiplatelet agents. Curr Med Chem 9:577–589
Bhatt DL, Fox KA, Hacke W, Berger PB, Black HR, Boden WE, Cacoub P, Cohen EA, Creager MA, Easton JD, Flather MD, Haffner SM, Hamm CW, Hankey GJ, Johnston SC, Mak KH, Mas JL, Montalescot G, Pearson TA, Steg PG, Steinhubl SR, Weber MA, Brennan DM, Fabry-Ribaudo L, Booth J, Topol EJ, for the CHARISMA Investigators (2006) Clopidogrel and aspirin versus aspirin alone for the prevention of atherothrombotic events. N Engl J Med 354:1706–1717
Yusuf S, Zhao F, Mehta SR, Chrolavicius S, Tognoni G, Fox KK, for the Clopidogrel in Unstable Angina to Prevent Recurrent Events Trial Investigators (2001) Effects of clopidogrel in addition to aspirin in patients with acute coronary syndromes without ST-segment elevation. N Engl J Med 345:494–502
Wiviott SD, Braunwald E, McCabe CH, Montalescot G, Ruzyllo W, Gottlieb S, Neumann FJ, Ardissino D, De Servi S, Murphy SA, Riesmeyer J, Weerakkody G, Gibson CM, Antman EM, for the TRITON-TIMI 38 Investigators (2007) Prasugrel versus clopidogrel in patients with acute coronary syndromes. N Engl J Med 357:2001–2015
Andersen H, Greenberg DL, Fujikawa K, Xu W, Chung DW, Davie EW (1999) Protease-activated receptor 1 is the primary mediator of thrombin-stimulated platelet procoagulant activity. Proc Natl Acad Sci USA 96:11189–11193
Chackalamannil S (2003) G-protein couple receptor antagonists-1: protease activated receptor-1 (PAR-1) antagonists as novel cardiovascular therapeutic agents. Curr Top Med Chem 3:1115–1123
Goto S, Yamaguchi T, Ikeda Y, Kato K, Yamaguchi H, Jensen P (2010) Safety and exploratory efficacy of the novel thrombin receptor (PAR-1) antagonist SCH530348 for non-ST-segment elevation acute coronary syndrome. J Atheroscler Thromb 17:156–164
Shinohara Y, Goto S, Doi M, Jensen P (2010) Safety of the novel protease-activated receptor-1 antagonist vorapaxar in Japanese patients with a history of ischemic stroke. J Stroke Cerebrovasc Dis. doi:10.1016/j.jstrokecerebrovasdis.2010.09.005
Becker RC, Moliterno DJ, Jennings LK, Pieper KS, Pei J, Niederman A, Ziada KM, Berman G, Strony J, Joseph D, Mahaffey KW, Van de Werf F, Veltri E, Harrington RA, for the TRA-PCI Investigators (2009) Safety and tolerability of SCH 530348 in patients undergoing non-urgent percutaneous coronary intervention: a randomised, double-blind, placebo-controlled phase II study. Lancet 373:919–928
Morrow DA, Scirica BM, Fox KA, Berman G, Strony J, Veltri E, Bonaca MP, Fish P, McCabe CH, Braunwald E, for the TRA 2°P-TIMI 50 Investigators (2009) Evaluation of a novel antiplatelet agent for secondary prevention in patients with a history of atherosclerotic disease: design and rationale for the Thrombin-Receptor Antagonist in Secondary Prevention of Atherothrombotic Ischemic Events (TRA 2°P)-TIMI 50 trial. Am Heart J 158:335–341
TRA*CER Executive and Steering Committees (2009) The thrombin receptor antagonist for clinical event reduction in acute coronary syndrome (TRA*CER) trial: study design and rationale. Am Heart J 158:327–334
Kosoglou T, Reyderman L, Tiessen RG, van Vliet AA, Fales RR, Keller R, Yang B, Cutler DL (2012) Pharmacodynamics and pharmacokinetics of the novel PAR-1 antagonist vorapaxar (formerly SCH 530348) in healthy subjects. Eur J Clin Pharmacol 68:249–258
Kosoglou T, Reyderman L, Kasserra C, Jennings LK, Young S, Xuan F, Pei J, Maxwell SE, Meehan AG, Cutler DL (2012) No differences in the pharmacodynamics and pharmacokinetics of the thrombin receptor antagonist vorapaxar between healthy Japanese and Caucasian subjects. Eur J Clin Pharmacol 68:291–300
Ghosal A, Lu X, Penner N, Gao L, Ramanathan R, Chowdhury SK, Kishnani NS, Alton KB (2011) Identification of human liver cytochrome P450 enzymes involved in the metabolism of SCH 530348 (vorapaxar), a potent oral thrombin protease-activated receptor 1 antagonist. Drug Metab Dispos 39:30–38
Statkevich P, Kosoglou T, Kumar B, Li J, Xuan F, Wang Z, Meehan AG, Cutler DL (2012) Pharmacokinetics of vorapaxar and its metabolite SCH 2046273 (M20) following oral administration in healthy Chinese and US subjects (abstract). Clin Pharmacol Ther (in press)
Verbeeck RK (2008) Pharmacokinetics and dosage adjustment in patients with hepatic dysfunction. Eur J Clin Pharmacol 64:1147–1161
Pugh RN, Murray-Lyon IM, Dawson JL, Pietroni MC, Williams R (1973) Transection of the oesophagus for bleeding oesophageal varices. Br J Surg 60:646–649
McLean AJ, Morgan DJ (1991) Clinical pharmacokinetics in patients with liver disease. Clin Pharmacokinet 21:42–69
Donelli MG, Zucchetti M, Munzone E, D’Incalci M, Crosignani A (1998) Pharmacokinetics of anticancer agents in patients with impaired liver function. Eur J Cancer 34:33–46
Sulsa GM, Atkinson AJ (2007) Effect of liver disease on pharmacokinetics. In: Atkinson AJ Jr, Abernethy DR, Daniels CE, Dedrick R, Markey SP (eds) Principles of clinical pharmacology, 2nd edn. Academic Press, New York, pp 73–87
Adedoyin A, Arns PA, Richards WO, Wilkinson GR, Branch RA (1998) Selective effect of liver disease on the activities of specific metabolizing enzymes: investigation of cytochromes P450 2C19 and 2D6. Clin Pharmacol Ther 64:8–17
Horak J, White J, Harris AL, Verrill M, Carmichael J, Holt A, Cantarini M, Macpherson M, Swaisland A, Swaisland H, Twelves C (2011) The effect of different etiologies of hepatic impairment on the pharmacokinetics of gefitinib. Cancer Chemother Pharmacol 68(6):1485–1495
Weil A, Martin P, Smith R, Oliver S, Langmuir P, Read J, Molz KH (2010) Pharmacokinetics of vandetanib in subjects with renal or hepatic impairment. Clin Pharmacokinet 49:607–618
Bruderer S, Detishin V, Tsvitbaum N, Dingemanse J (2011) Influence of different degrees of liver impairment on the pharmacokinetics of clazosentan. Br J Clin Pharmacol 71:52–60
Zhang C, Thabut D, Kamath PS, Shah VH (2011) Oesophageal varices in cirrhotic patients: from variceal screening to primary prophylaxis of the first oesophageal variceal bleeding. Liver Int 31:108–119
Acknowledgments
The authors wish to thank Laura Black, Jing Sun, and Kristen Lewis (currently or formerly of Merck Sharp & Dohme Corp., a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ, USA) for assistance in the execution of this study and the preparation of this manuscript for publication.
Conflict of interest
P. Statkevich, T. Kosoglou, B. Kumar, F. Xuan, C. Trusley, J.E. Schiller, R.B. Langdon, and D.L. Cutler all declare that they are full-time employees of Merck Sharp & Dohme Corp. (a subsidiary of Merck & Co., Inc., Whitehouse Station, NJ, USA) or were full-time employees of Schering-Plough Corporation (now Merck Sharp & Dohme Corp.) at the time of the study, and may own stock or hold stock options in Merck & Co., Inc. R.A. Preston received research grant support from Schering-Plough Corporation for the conduct of this study and is a full-time employee of the University of Miami School of Medicine, which received funding from Schering-Plough Corporation.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Statkevich, P., Kosoglou, T., Preston, R.A. et al. Pharmacokinetics of the novel PAR-1 antagonist vorapaxar in patients with hepatic impairment. Eur J Clin Pharmacol 68, 1501–1508 (2012). https://doi.org/10.1007/s00228-012-1269-7
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-012-1269-7