Abstract
Purpose
Drug-induced immune thrombocytopaenia is a rare, serious condition that can be triggered by numerous medications. To characterize the spectrum of drugs associated with immune thrombocytopaenia (ITP) in the Berlin Case–Control Surveillance Study (FAKOS).
Methods
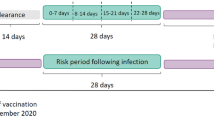
Adult hospitalized patients with new onset idiopathic, secondary or drug-induced acute ITP and hospital control patients were ascertained by active surveillance in 50 Berlin hospitals (>180 clinical departments) between 2000 and 2009. Drug exposures were obtained in a personal interview. Chronic cases were excluded in a follow-up after 6 or more months. A standardized causality assessment was conducted for each ITP patient to assess possible drug aetiology. Drug risks were quantified in a case–control design with unconditional logistic regression analysis.
Results
Ninety out of 169 validated cases of acute ITP were assessed as being at least possibly drug-related (n = 85 different drugs overall, n = 30 drugs with certain or probable causality). Drugs involved in ≥2 cases with a probable or certain relationship were tirofiban (n = 10 cases), abciximab (n = 4), trimethoprim/sulphamethoxazole (n = 4), influenza vaccine (n = 3), and citalopram (n = 2). Pneumococcal and poliomyelitis vaccine were assessed as probably causing ITP in one case each. In the case–control analyses, significantly increased risks were observed for tirofiban, abciximab, trimethoprim/sulphamethoxazole, gentamicin, triamterene/hydrochlorothiazide, drospirenone/ethinylestradiol, and influenza vaccination.
Conclusions
Our study confirms known ITP risks for glycoprotein IIb/IIIa receptor antagonists and sulphonamides and generates signals for several other drugs and vaccines. New onset of ITP should not only direct attention to drugs as possible aetiological agents, but also to vaccines that are known to cause autoimmune phenomena.

Similar content being viewed by others
References
Aster RH, Bougie DW (2007) Drug-induced immune thrombocytopenia. N Engl J Med 357(6):580–587
Kenney B, Stack G (2009) Drug-induced thrombocytopenia. Arch Pathol Lab Med 133(2):309–314
Kang SY, Choi JC, Kang JH, Lee JS (2010) Acute subdural hemorrhage associated with rifampicin-induced thrombocytopenia. Neurol Sci 31(2):199–200
Kolluri VR, Reddy DR, Reddy PK, Naidu MR, Kumari CS (1986) Subdural hematoma secondary to immune thrombocytopenic purpura: case report. Neurosurgery 19(4):635–636
Single report database for drug–induced thrombocytopenia: An update. http://www.ouhsc.edu/platelets/InternetPostingLitSingleFrames2_18_11.htm. Accessed 13 September 2011
George JN, Aster RH (2009) Drug-induced thrombocytopenia: pathogenesis, evaluation, and management. Hematol Am Soc Hematol Educ Program:153–158
Visentin GP, Liu CY (2007) Drug-induced thrombocytopenia. Hematol Oncol Clin North Am 21(4):685–696, vi
Van den Bemt PM, Meyboom RH, Egberts AC (2004) Drug-induced immune thrombocytopenia. Drug Saf 27(15):1243–1252
Database for drug–induced thrombocytopenia from Group Patient Reports: an update. http://www.ouhsc.edu/platelets/InternetPostingLitGroup2_18_11.htm. Accessed 13 September 2011
George JN, Raskob GE, Shah SR, Rizvi MA, Hamilton SA, Osborne S, Vondracek T (1998) Drug-induced thrombocytopenia: a systematic review of published case reports. Ann Intern Med 129(11):886–890
Aster RH, Curtis BR, McFarland JG, Bougie DW (2009) Drug-induced immune thrombocytopenia: pathogenesis, diagnosis, and management. J Thromb Haemost 7(6):911–918
Reese JA, Li X, Hauben M, Aster RH, Bougie DW, Curtis BR, George JN, Vesely SK (2010) Identifying drugs that cause acute thrombocytopenia: an analysis using 3 distinct methods. Blood 116(12):2127–2133
Warkentin TE, Anderson JA (2010) DITP causation: 3 methods better than 1? Blood 116(12):2002–2003
Kaufman DW, Kelly JP, Johannes CB, Sandler A, Harmon D, Stolley PD, Shapiro S (1993) Acute thrombocytopenic purpura in relation to the use of drugs. Blood 82(9):2714–2718
ten Berg MJ, Huisman A, Souverein PC, Schobben AF, Egberts AC, van Solinge WW, van den Bemt PM (2006) Drug-induced thrombocytopenia: a population study. Drug Saf 29(8):713–721
Bertuola F, Morando C, Menniti-Ippolito F, Da Cas R, Capuano A, Perilongo G, Da Dalt L (2010) Association between drug and vaccine use and acute immune thrombocytopenia in childhood: a case–control study in Italy. Drug Saf 33(1):65–72
Andersohn F, Bronder E, Klimpel A, Garbe E (2004) Proportion of drug-related serious rare blood dyscrasias: estimates from the Berlin Case–control Surveillance Study. Am J Hematol 77(3):316–318
George JN, Woolf SH, Raskob GE, Wasser JS, Aledort LM, Ballem PJ, Blanchette VS, Bussel JB, Cines DB, Kelton JG, Lichtin AE, McMillan R, Okerbloom JA, Regan DH, Warrier I (1996) Idiopathic thrombocytopenic purpura: a practice guideline developed by explicit methods for the American Society of Hematology. Blood 88(1):3–40
The Uppsala Monitoring Centre. The use of the WHO-UMC system for standardised case causality assessment. http://www.who-umc.org/Graphics/24734.pdf. Accessed 13 September 2011
World Health Organization. The Anatomical Therapeutic Chemical Classification System with Defined Daily Doses (ATC/DDD). http://www.who.int/classifications/atcddd/en/. Accessed 13 September 2011
Kiefel V, Santoso S, Weisheit M, Mueller-Eckhardt C (1987) Monoclonal antibody–specific immobilization of platelet antigens (MAIPA): a new tool for the identification of platelet-reactive antibodies. Blood 70(6):1722–1726
Kiefel V (1992) The MAIPA assay and its applications in immunohaematology. Transfus Med 2(3):181–188
Meyer O, Gaedicke G, Salama A (1999) Demonstration of drug-dependent antibodies in two patients with neutrophenia and successful treatment with granulocyte-colony-stimulating factor. Transfusion 39(5):527–530
Schwabe U, Paffrath D (2009) Arzneiverordnungsreport 2009. Springer Medizin Verlag, Heidelberg
Said SM, Hahn J, Schleyer E, Muller M, Fiedler GM, Buerke M, Prondzinsky R (2007) Glycoprotein IIb/IIIa inhibitor-induced thrombocytopenia: diagnosis and treatment. Clin Res Cardiol 96(2):61–69
Curtis BR, Swyers J, Divgi A, McFarland JG, Aster RH (2002) Thrombocytopenia after second exposure to abciximab is caused by antibodies that recognize abciximab-coated platelets. Blood 99(6):2054–2059
Jubelirer SJ, Koenig BA, Bates MC (1999) Acute profound thrombocytopenia following C7E3 Fab (Abciximab) therapy: case reports, review of the literature and implications for therapy. Am J Hematol 61(3):205–208
Vahdat B, Canavy I, Fourcade L, Garcia E, Quilici J, Bonnet JL, Bory M (2000) Fatal cerebral hemorrhage and severe thrombocytopenia during abciximab treatment. Catheter Cardiovasc Interv 49(2):177–180
Coons JC, Barcelona RA, Freedy T, Hagerty MF (2005) Eptifibatide-associated acute, profound thrombocytopenia. Ann Pharmacother 39(2):368–372
Curtis BR, Divgi A, Garritty M, Aster RH (2004) Delayed thrombocytopenia after treatment with abciximab: a distinct clinical entity associated with the immune response to the drug. J Thromb Haemost 2(6):985–992
Nurden P, Clofent-Sanchez G, Jais C, Bermejo E, Leroux L, Coste P, Nurden AT (2004) Delayed immunologic thrombocytopenia induced by abciximab. Thromb Haemost 92(4):820–828
Dickson HG (1978) Trimethoprim-sulphamethoxazole and thrombocytopenia. Med J Aust 2(1):5–7
Keisu M, Wiholm BE, Palmblad J (1990) Trimethoprim-sulphamethoxazole-associated blood dyscrasias. Ten years' experience of the Swedish spontaneous reporting system. J Intern Med 228(4):353–360
Rothman KJ (1981) Induction and latent periods. Am J Epidemiol 114(2):253–259
Leach MF, Aubuchon JP (1999) Frequency of thrombocytopenia associated with gentamicin therapy. Immunohematology 15(4):167–170
Wraith DC, Goldman M, Lambert PH (2003) Vaccination and autoimmune disease: what is the evidence? Lancet 362(9396):1659–1666
Jadavji T, Scheifele D, Halperin S (2003) Thrombocytopenia after immunization of Canadian children, 1992 to 2001. Pediatr Infect Dis J 22(2):119–122
Casoli P, Tumiati B (1989) Acute idiopathic thrombocytopenic purpura after anti-influenza vaccination. Medicina (Firenze) 9(4):417–418
Ikegame K, Kaida K, Fujioka T, Kawakami M, Hasei H, Inoue T, Taniguchi Y, Yoshihara S, Hayashi S, Kurata Y, Ogawa H (2006) Idiopathic thrombocytopenic purpura after influenza vaccination in a bone marrow transplantation recipient. Bone Marrow Transplant 38(4):323–324
Tishler M, Levy O, Amit-Vazina M (2006) Immune thrombocytopenic purpura following influenza vaccination. Isr Med Assoc J 8(5):322–323
Wagner K (1964) Protrahierte Thrombozytopenie nach Polymyelitis Schutzimpfung. Wien Med Wochenschr 114:628–631
Wise RP, Iskander J, Pratt RD, Campbell S, Ball R, Pless RP, Braun MM (2004) Postlicensure safety surveillance for 7-valent pneumococcal conjugate vaccine. JAMA 292(14):1702–1710
Altintas E, Oguz D, Kacar S, Ozderin Y, Sezgin O, Zengin NI (2004) Dydrogesterone-induced hepatitis and autoimmune hemolytic anemia. Turk J Gastroenterol 15(1):49–52
Kacar S, Akdogan M, Kosar Y, Parlak E, Sasmaz N, Oguz P, Aydog G (2002) Estrogen and cyproterone acetate combination-induced autoimmune hepatitis. J Clin Gastroenterol 35(1):98–100
Acknowledgements
Cases were collected within the study “Berlin Case–Control Surveillance (FAKOS) of Serious Blood Dyscrasias”, which is supported by a grant from the Federal Institute for Drugs and Medical Devices (Bonn, Germany). We wish to thank all the hospitals that contributed cases and controls to this study. A list of all the hospitals is provided in a separate Appendix.
Conflict of interest
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Appendix: list of all hospitals contributing cases and controls to this study
Appendix: list of all hospitals contributing cases and controls to this study
Bundeswehrkrankenhaus, Caritas-Klinik Maria Heimsuchung, Charité Universitätsmedizin Berlin Campus Benjamin Franklin, Charité Universitätsmedizin Berlin Campus Mitte, Charité Universitätsmedizin Berlin Campus Virchow-Klinikum, Deutsches Herzzentrum Berlin (DHZB), Dominikus-Krankenhaus, DRK Kliniken Berlin-Köpenick, DRK Kliniken Berlin-Mitte, DRK Kliniken Berlin-Westend, Evangelische Elisabeth-Klinik, Evangelisches Geriatriezentrum Berlin (EGZB), Evangelisches Krankenhaus Hubertus, Evangelisches Krankenhaus Königin Elisabeth-Herzberge, Evangelisches Waldkrankenhaus, Evangelische Lungenklinik Berlin, Franziskus-Krankenhaus, Friedrich von Bodelschwingh-Klinik, Gemeinschaftskrankenhaus Havelhöhe, HELIOS Klinikum Berlin Buch, HELIOS Zentralklinik Emil von Behring, Immanuel-Krankenhaus Rheumaklinik Berlin-Wannsee, Immanuel-Krankenhaus Rheumaklinik Berlin-Buch, Jüdisches Krankenhaus, Kliniken im Theodor-Wenzel-Werk, Krankenhaus Bethel, Krankenhaus Hedwigshöhe, Krankenhaus des Maßregelvollzugs, Krankenhaus Waldfriede, Malteser Krankenhaus, Martin-Luther Krankenhaus, Park-Klinik Weißensee, Paulinenkrankenhaus, Sana Klinikum Lichtenberg, Schlosspark-Klinik, St. Gertrauden Krankenhaus, St. Hedwig Krankenhaus, St. Joseph-Krankenhaus, St. Joseph-Krankenhaus Berlin-Weißensee, St. Marien Krankenhaus, Unfallkrankenhaus Berlin, Vivantes Auguste-Viktoria Klinikum, Vivantes Humboldt Klinikum, Vivantes Klinikum am Urban, Vivantes Klinikum im Friedrichshain, Vivantes Klinikum Hellersdorf, Vivantes Klinikum Neukölln, Vivantes Klinikum Prenzlauer Berg, Vivantes Klinikum Spandau, Vivantes Wenckebach-Klinikum, Wichern Krankenhaus
Rights and permissions
About this article
Cite this article
Garbe, E., Andersohn, F., Bronder, E. et al. Drug-induced immune thrombocytopaenia: results from the Berlin Case–Control Surveillance Study. Eur J Clin Pharmacol 68, 821–832 (2012). https://doi.org/10.1007/s00228-011-1184-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-011-1184-3