Abstract
Purpose
Cytochrome P450 2D6 (CYP2D6) genotypes and the dextromethorphan/dextrorphan (DXM/DXT) metabolic ratio (MR), which is a marker of CYP2D6 activity, were studied in 118 unrelated healthy Ecuadorians.
Methods
Genotyping of CYP2D6 was performed by amplification of entire CYP2D6 gene by XL-PCR for CYP2D6*5 and multiplication alleles and by real time-PCR for CYP2D6 *2, *3, *4, *6, *10, *17, *29, *35, *41, and copy number. The plasma levels of DXM and its metabolite DXT were determined on a high-performance liquid chromatography–UV system.
Results
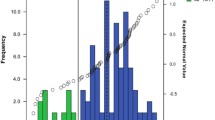
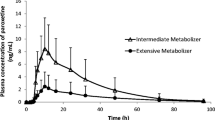
The proportions of non-functional alleles were 0.4, 10.6, 0.8, 2.1, and 0% for CYP2D6*3, *4, *4 × N, *5, and *6, respectively. Genotypically, only one of the subjects (0.9%) was homozygous for two inactive alleles and phenotypically classified as a poor metabolizer (PM). The MRs (mean ± standard deviation) corresponding to “activity scores” of 0, 0.5, 1, 1.5, 2, and 2.5 were 10.57 (n = 1), 1.63 ± 0.35 (n = 2), 1.16 ± 0.74 (n = 29), 1.00 ± 0.47 (n = 8), 1.24 ± 0.82 (n = 76), and 1.30 ± 0.32 (n = 2), respectively.
Conclusions
Our data suggest that only 1% of subjects of this Ecuadorian population were PMs and that none were phenotypically ultrarapid metabolizers, which is in agreement with previous findings in other Amerindian populations.


Similar content being viewed by others
References
Ingelman-Sundberg M (2005) Genetic polymorphisms of cytochrome P450 2D6 (CYP2D6): clinical consequences, evolutionary aspects and functional diversity. Pharmacogenomics J 5:6–13
CYP2D6 allele nomenclature. Available at: http://www.cypalleles.ki.se/cyp2d6.htm. Accessed 17 June 2011
LLerena A, Dorado P, Peñas-Lledó EM (2009) Pharmacogenetics of debrisoquine and its use as a marker for CYP2D6 hydroxylation capacity. Pharmacogenomics 10:17–28
Frank D, Jaehde U, Fuhr U (2007) Evaluation of probe drugs and pharmacokinetic metrics for CYP2D6 phenotyping. Eur J Clin Pharmacol 63:321–333
Tamminga WJ, Wemer J, Oosterhuis B, Brakenhoff JP, Gerrits MG, de Zeeuw RA, de Leij LF, Jonkman JH (2001) An optimized methodology for combined phenotyping and genotyping on CYP2D6 and CYP2C19. Eur J Clin Pharmacol 57:143–146
Dorado P, Suarez-Kurtz G, LLerena A (2007) Pharmacogenetics of cytochrome P450 in Hispanic populations. In: Suarez-Kurtz G (ed) Pharmacogenomics in admixed populations. Landes Bioscience, Austin, pp 60–74
LLerena A, Dorado P, Ramírez R, González I, Alvarez M, Peñas-Lledó EM, Pérez B, Calzadilla LR (2010) CYP2D6 genotype and debrisoquine hydroxylation phenotype in Cubans and Nicaraguans. Pharmacogenomics J. doi:10.1038/tpj.2010.85
Dorado P, Cáceres M, Pozo-Guisado E, Wong ML, Licinio J, Llerena A (2005) Development of a PCR-based strategy for CYP2D6 genotyping including gene multiplication of worldwide potential use. Biotechniques 39[10 Suppl]:S571–S574
Gaedigk A, Simon SD, Pearce RE, Bradford LD, Kennedy MJ, Leeder JS (2008) The CYP2D6 activity score: translating genotype information into a qualitative measure of phenotype. Clin Pharmacol Ther 83:234–242
Arias TD, Jorge LF, Lee D, Barrantes R, Inaba T (1988) The oxidative metabolism of sparteine in the Cuna Amerindians of Panama: absence of evidence for deficient metabolizers. Clin Pharmacol Ther 43:456–465
Sosa-Macías M, Elizondo G, Flores-Pérez C, Flores-Pérez J, Bradley-Alvarez F, Alanis-Bañuelos RE, Lares-Asseff I (2006) CYP2D6 genotype and phenotype in Amerindians of Tepehuano origin and Mestizos of Durango, Mexico. J Clin Pharmacol 46:527–536
Yeh GC, Tao PL, Ho HO, Lee YJ, Chen JY, Sheu MT (2003) Analysis of pharmacokinetic parameters for assessment of dextromethorphan metabolic phenotypes. J Biomed Sci 10:552–564
Chládek J, Zimová G, Beránek M, Martínková J (2000) In-vivo indices of CYP2D6 activity: comparison of dextromethorphan metabolic ratios in 4-h urine and 3-h plasma. Eur J Clin Pharmacol 56:651–657
Muñoz S, Vollrath V, Vallejos MP, Miquel JF, Covarrubias C, Raddatz A, Chianale J (1998) Genetic polymorphisms of CYP2D6, CYP1A1 and CYP2E1 in the South-Amerindian population of Chile. Pharmacogenetics 8:343–351
Sosa-Macías M, Dorado P, Alanis-Bañuelos RE, LLerena A, Lares-Asseff I (2010) Influence of CYP2D6 deletion, multiplication, −1584 C>G, 31 G>A and 2988 G>A gene polymorphisms on dextromethorphan metabolism among Mexican tepehuanos and mestizos. Pharmacology 86:30–36
Suarez-Kurtz G, Pena SD (2006) Pharmacogenomics in the Americas: the impact of genetic admixture. Curr Drug Targets 7:1649–1658
Kohlrausch FB, Gama CS, Lobato MI, Belmonte-de-Abreu P, Gesteira A, Barros F, Carracedo A, Hutz MH (2009) Molecular diversity at the CYP2D6 locus in healthy and schizophrenic southern Brazilians. Pharmacogenomics 10:1457–1466
Isaza CA, Henao J, López AM, Cacabelos R (2000) Isolation, sequence and genotyping of the drug metabolizer CYP2D6 gene in the Colombian population. Methods Find Exp Clin Pharmacol 22:695–705
Jorge LF, Eichelbaum M, Griese EU, Inaba T, Arias TD (1999) Comparative evolutionary pharmacogenetics of CYP2D6 in Ngawbe and Embera Amerindians of Panama and Colombia: role of selection versus drift in world populations. Pharmacogenetics 9:217–228
Mendoza R, Wan YJ, Poland RE, Smith M, Zheng Y, Berman N, Lin KM (2001) CYP2D6 polymorphism in a Mexican American population. Clin Pharmacol Ther 70:552–560
Luo HR, Gaedigk A, Aloumanis V, Wan YJ (2005) Identification of CYP2D6 impaired functional alleles in Mexican Americans. Eur J Clin Pharmacol 61:797–802
Casner PR (2005) The effect of CYP2D6 polymorphisms on dextromethorphan metabolism in Mexican Americans. J Clin Pharmacol 45:1230–1235
López M, Guerrero J, Jung-Cook H, Alonso ME (2005) CYP2D6 genotype and phenotype determination in a Mexican Mestizo population. Eur J Clin Pharmacol 61:749–754
LLerena A, Edman G, Cobaleda J, Benítez J, Schalling D, Bertilsson L (1993) Relationship between personality and debrisoquine hydroxylation capacity. Suggestion of an endogenous neuroactive substrate or product of the cytochrome P4502D6. Acta Psychiatr Scand 87:23–28
Estevez F, Giusti M, Parrillo S, Oxandabarat J (1997) Dextromethorphan O-demethylation polymorphism in the Uruguayan population. Eur J Clin Pharmacol 52:417–418
Acknowledgments
The technical assistance of Inés López and Beatriz Grillo is gratefully acknowledged. We also thank to Eva M. Peñas-Lledó PhD for her fruitful comments and review. This study was partially funded by AEXCID Cooperación Extremeña of Junta de Extremadura (9IA006) and coordinated in the RIBEF network (Red Iberoamericana de Farmacogenética y Farmacogenómica; www.ribef.com). It was also partly supported by the Institute of Health Carlos III-FIS and European Union (FEDER) Grants PI10/02758, PI10/02010, and CP06/00030 (P. Dorado).
Conflict of interest statement
The authors declare that they have no conflict of interest.
Author information
Authors and Affiliations
Corresponding authors
Rights and permissions
About this article
Cite this article
Dorado, P., Heras, N., Machín, E. et al. CYP2D6 genotype and dextromethorphan hydroxylation phenotype in an Ecuadorian population. Eur J Clin Pharmacol 68, 637–644 (2012). https://doi.org/10.1007/s00228-011-1147-8
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-011-1147-8