Abstract
Purpose
The main metabolic pathways of oxycodone, a potent opioid analgetic, are N-demethylation (CYP3A4) to inactive noroxycodone and O-demethylation (CYP2D6) to active oxymorphone. We performed a three-way, placebo-controlled, double-blind cross-over study to assess the pharmacokinetic and pharmacodynamic consequences of drug interactions with oxycodone.
Methods
The 12 participants (CYP2D6 extensive metabolizers) were pre-treated with placebo, ketoconazole or paroxetine before oral oxycodone ingestion (0.2 mg/kg).
Results
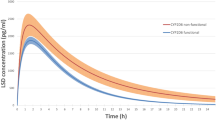
Pre-treatment with ketoconazole increased the AUC for oxycodone 2- to 3-fold compared with placebo or paroxetine. In combination with placebo, oxycodone induced the expected decrease in pupil diameter. This decrease was accentuated in the presence of ketoconazole, but blunted by paroxetine. In comparison to pre-treatment with placebo, ketoconazole increased nausea, drowsiness, and pruritus associated with oxycodone. In contrast, the effect of pre-treatment with paroxetine on the above-mentioned adverse events was not different from that of placebo. Ketoconazole increased the analgetic effect of oxycodone, whereas paroxetine was not different from placebo.
Conclusions
Inhibition of CYP3A4 by ketoconazole increases the exposure and some pharmacodynamic effects of oxycodone. Paroxetine pretreatment inhibits CYP2D6 without inducing relevant changes in oxycodone exposure, and partially blunts the pharmacodynamic effects of oxycodone due to intrinsic pharmacological activities. Pharmacodynamic changes associated with CYP3A4 inhibition may be clinically important in patients treated with oxycodone.





Similar content being viewed by others
References
Davis MP, Varga J, Dickerson D, Walsh D, LeGrand SB, Lagman R (2003) Normal-release and controlled-release oxycodone: pharmacokinetics, pharmacodynamics, and controversy. Support Care Cancer 11:84–92
Nuutinen LS, Wuolijoki E, Pentikainen IT (1986) Diclofenac and oxycodone in treatment of postoperative pain: a double-blind trial. Acta Anaesthesiol Scand 30:620–624
Parris WC, Johnson BW Jr, Croghan MK et al (1998) The use of controlled-release oxycodone for the treatment of chronic cancer pain: a randomized, double-blind study. J Pain Symptom Manage 16:205–211
Bruera E, Belzile M, Pituskin E et al (1998) Randomized, double-blind, cross-over trial comparing safety and efficacy of oral controlled-release oxycodone with controlled-release morphine in patients with cancer pain. J Clin Oncol 16:3222–3229
Curtis GB, Johnson GH, Clark P et al (1999) Relative potency of controlled-release oxycodone and controlled-release morphine in a postoperative pain model. Eur J Clin Pharmacol 55:425–429
Kirvela M, Lindgren L, Seppala T, Olkkola KT (1996) The pharmacokinetics of oxycodone in uremic patients undergoing renal transplantation. J Clin Anesth 8:13–18
Lalovic B, Phillips B, Risler LL, Howald W, Shen DD (2004) Quantitative contribution of CYP2D6 and CYP3A to oxycodone metabolism in human liver and intestinal microsomes. Drug Metab Dispos 32:447–454
Inturrisi CE (2002) Clinical pharmacology of opioids for pain. Clin J Pain 18:S3–S13
Heiskanen T, Olkkola KT, Kalso E (1998) Effects of blocking CYP2D6 on the pharmacokinetics and pharmacodynamics of oxycodone. Clin Pharmacol Ther 64:603–611
Lalovic B, Kharasch E, Hoffer C, Risler L, Liu-Chen LY, Shen DD (2006) Pharmacokinetics and pharmacodynamics of oral oxycodone in healthy human subjects: role of circulating active metabolites. Clin Pharmacol Ther 79:461–479
Zwisler ST, Enggaard TP, Noehr-Jensen L et al (2009) The hypoalgesic effect of oxycodone in human experimental pain models in relation to the CYP2D6 oxidation polymorphism. Basic Clin Pharmacol Toxicol 104:335–344
Zwisler ST, Enggaard TP, Mikkelsen S, Brosen K, Sindrup SH (2010) Impact of the CYP2D6 genotype on post-operative intravenous oxycodone analgesia. Acta Anaesthesiol Scand 54:232–240
Hagelberg NM, Nieminen TH, Saari TI, Neuvonen M, Neuvonen PJ, Laine K, Olkkola KT (2009) Voriconazole drastically increases exposure to oral oxycodone. Eur J Clin Pharmacol 65:263–271
Gronlund J, Saari T, Hagelberg N, Martikainen IK, Neuvonen PJ, Olkkola KT, Laine K (2010) Effect of telithromycin on the pharmacokinetics and pharmacodynamics of oral oxycodone. J Clin Pharmacol 50:101–108
Nieminen TH, Hagelberg NM, Saari TI et al (2009) Rifampin greatly reduces the plasma concentrations of intravenous and oral oxycodone. Anesthesiology 110:1371–1378
Laugesen S, Enggaard TP, Pedersen RS, Sindrup SH, Brosen K (2005) Paroxetine, a cytochrome P450 2D6 inhibitor, diminishes the stereoselective O-demethylation and reduces the hypoalgesic effect of tramadol. Clin Pharmacol Ther 77:312–323
Müller B, Zopf K, Bachofer J, Steimer W (2003) Optimized strategy for rapid cytochrome P450 2D6 genotyping by real-time long PCR. Clin Chem 49:1624–1631
Wenk M, Todesco L, Krahenbuhl S (2004) Effect of St John’s wort on the activities of CYP1A2, CYP3A4, CYP2D6, N-acetyltransferase 2, and xanthine oxidase in healthy males and females. Br J Clin Pharmacol 57:495–499
Neuvonen M, Neuvonen PJ (2008) Determination of oxycodone, noroxycodone, oxymorphone, and noroxymorphone in human plasma by liquid chromatography-electrospray-tandem mass spectrometry. Ther Drug Monit 30:333–340
Haegeli L, Brunner-La Rocca HP, Wenk M, Pfisterer M, Drewe J, Krahenbuhl S (2007) Sublingual administration of furosemide: new application of an old drug. Br J Clin Pharmacol 64:804–809
Savitzky A, Golay MJE (2002) Smoothing and differentiation of data by simplified least squares procedures. Anal Chem 36:1627–1639
Eisenberg E, Cohen D, Lawental E, Pud D (2007) Personality traits and sensitivity to pain in male chronic opioid addicts. J Opioid Manag 3:225–230
Grönlund J, Saari TI, Hagelberg NM, Neuvonen PJ, Olkkola KT, Laine K (2010) Exposure to oral oxycodone is increased by concomitant inhibition of CYP2D6 and 3A4 pathways, but not by inhibition of CYP2D6 alone. Br J Clin Pharmacol 70:78–87
Lemberg KK, Heiskanen TE, Neuvonen M, Kontinen VK, Neuvonen PJ, Dahl ML, Kalso EA (2010) Does co-administration of paroxetine change oxycodone analgesia: an interaction study in chronic pain patients. Scand J Pain 1:24–33
Johnson AM (1989) An overview of the animal pharmacology of paroxetine. Acta Psychiatr Scand Suppl 350:14–20
Larson M, Folstein S (2000) Selective serotonin reuptake inhibitor-induced mydriasis. J Am Acad Child Adolesc Psychiatry 39:138–139
Schmitt JA, Riedel WJ, Vuurman EF, Kruizinga M, Ramaekers JG (2002) Modulation of the critical flicker fusion effects of serotonin reuptake inhibitors by concomitant pupillary changes. Psychopharmacology (Berl) 160:381–386
Knaggs RD, Crighton IM, Cobby TF, Fletcher AJ, Hobbs GJ (2004) The pupillary effects of intravenous morphine, codeine, and tramadol in volunteers. Anesth Analg 99:108–112
Murray RB, Adler MW, Korczyn AD (1983) The pupillary effects of opioids. Life Sci 33:495–509
Lee HK, Wang SC (1975) Mechanism of morphine-induced miosis in the dog. J Pharmacol Exp Ther 192:415–431
Prow MR, Martin KF, Heal DJ (1996) 8-OH-DPAT-induced mydriasis in mice: a pharmacological characterisation. Eur J Pharmacol 317:21–28
Duman EN, Kesim M, Kadioglu M, Yaris E, Kalyoncu NI, Erciyes N (2004) Possible involvement of opioidergic and serotonergic mechanisms in antinociceptive effect of paroxetine in acute pain. J Pharmacol Sci 94:161–165
Kesim M, Duman EN, Kadioglu M, Yaris E, Kalyoncu NI, Erciyes N (2005) The different roles of 5-HT(2) and 5-HT(3) receptors on antinociceptive effect of paroxetine in chemical stimuli in mice. J Pharmacol Sci 97:61–66
Singh VP, Jain NK, Kulkarni SK (2001) On the antinociceptive effect of fluoxetine, a selective serotonin reuptake inhibitor. Brain Res 915:218–226
Erjavec MK, Coda BA, Nguyen Q, Donaldson G, Risler L, Shen DD (2000) Morphine-fluoxetine interactions in healthy volunteers: analgesia and side effects. J Clin Pharmacol 40:1286–1295
Gordon NC, Heller PH, Gear RW, Levine JD (1994) Interactions between fluoxetine and opiate analgesia for postoperative dental pain. Pain 58:85–88
Ferrari A, Coccia CP, Bertolini A, Sternieri E (2004) Methadone—metabolism, pharmacokinetics and interactions. Pharmacol Res 50:551–559
Tateishi T, Krivoruk Y, Ueng YF, Wood AJ, Guengerich FP, Wood M (1996) Identification of human liver cytochrome P-450 3A4 as the enzyme responsible for fentanyl and sufentanil N-dealkylation. Anesth Analg 82:167–172
Kobayashi K, Yamamoto T, Chiba K, Tani M, Shimada N, Ishizaki T, Kuroiwa Y (1998) Human buprenorphine N-dealkylation is catalyzed by cytochrome P450 3A4. Drug Metab Dispos 26:818–821
Liu YT, Hao HP, Liu CX, Wang GJ, Xie HG (2007) Drugs as CYP3A probes, inducers, and inhibitors. Drug Metab Rev 39:699–721
Link B, Haschke M, Grignaschi N, Bodmer M, Aschmann YZ, Wenk M, Krahenbuhl S (2008) Pharmacokinetics of intravenous and oral midazolam in plasma and saliva in humans: usefulness of saliva as matrix for CYP3A phenotyping. Br J Clin Pharmacol 66:473–484
Kirchheiner J, Schmidt H, Tzvetkov M, Keulen JT, Lotsch J, Roots I, Brockmoller J (2007) Pharmacokinetics of codeine and its metabolite morphine in ultra-rapid metabolizers due to CYP2D6 duplication. Pharmacogenomics J 7:257–265
Koren G, Cairns J, Chitayat D, Gaedigk A, Leeder SJ (2006) Pharmacogenetics of morphine poisoning in a breastfed neonate of a codeine-prescribed mother. Lancet 368:704
Stamer UM, Stuber F, Muders T, Musshoff F (2008) Respiratory depression with tramadol in a patient with renal impairment and CYP2D6 gene duplication. Anesth Analg 107:926–929
Acknowledgements
This study was funded by the Division of Clinical Pharmacology & Toxicology, University Hospital Basel, Switzerland, and by Mundipharma Medical Company. We thank Natalie Grignaschi and Beatrice Vetter for the determination of oxycodone.
Author information
Authors and Affiliations
Corresponding author
Additional information
Oliver Kummer and Felix Hammann contributed equally to this work.
Rights and permissions
About this article
Cite this article
Kummer, O., Hammann, F., Moser, C. et al. Effect of the inhibition of CYP3A4 or CYP2D6 on the pharmacokinetics and pharmacodynamics of oxycodone. Eur J Clin Pharmacol 67, 63–71 (2011). https://doi.org/10.1007/s00228-010-0893-3
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00228-010-0893-3